Discover what CAR-T Cell Therapy is and how MedStar Health can help
The Stem Cell Transplant and Cellular Immunotherapy program at MedStar Georgetown is the only program in the Washington, D.C., metropolitan area to offer chimeric antigen receptor (CAR) T-cell therapy for adults. CAR T-cell clinical trials are ongoing in the U.S. and commercially approved treatment is available.
Our Stem Cell Transplant and Cellular Immunotherapy program offers six FDA approved CAR T-Cell therapies (YESCARTA®, KYMRIAH®, TECARTUS®, ABECMA®, Breyanzi®, and Carvykti®) to treat certain types of blood disorders and cancers.
CAR T-cell therapy involves collecting immune cells from your blood, modifying them in a lab, and returning the modified cells to you via an IV infusion. The goal is to stimulate your immune system to more effectively target cancer cells and eliminate disease.
The U.S. Food and Drug Administration has approved CAR T-cell therapy to treat adult patients with certain types of B-Cell Lymphoma that have not responded to other therapies or who have relapsed after two other kinds of treatment.
What to expect during CAR T-cell therapy
Before treatment
At your first visit, you will meet with your care team to:
-
Determine if you are an appropriate candidate to receive CAR T-cell therapy
-
Discuss your treatment approach
-
Discuss the CAR T-cell therapy process, potential side effects, and outcomes
-
Understand the impact on your quality of life and day-to-day activities
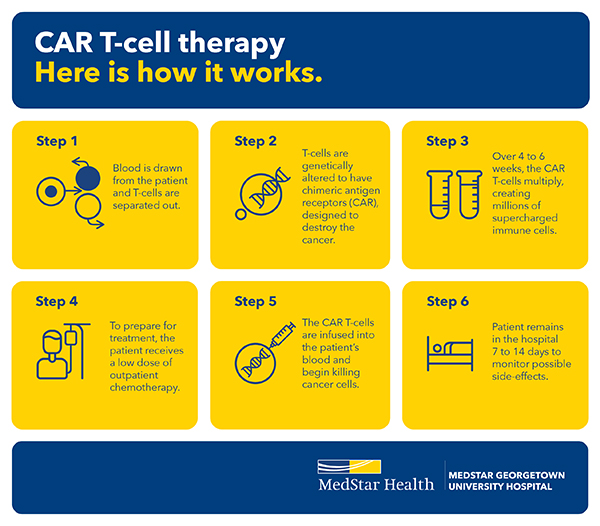
CAR T-cell therapy begins with a process involving blood collection, to obtain immune cells needed for CAR T-cell manufacturing.
Your blood will be collected by an Apheresis machine. The machine will sort your T-cells from your blood. The non-T cells are returned to your body. This procedure can take up to six hours and is performed on an outpatient basis.
Your T cells are then sent to a lab to be genetically modified. The chimeric antigen receptor, or CAR, is attached to the T-cells. The CAR(s) grow and expand in the lab and are ready to be re-infused into your body in approximately three weeks.
During CAR-T cell therapy
- You will receive chemotherapy to prepare your body to receive the modified CAR T-cells. Your chemotherapy regimen may be given on an outpatient or inpatient basis.
- Your CAR T-cells will be infused back to you after receiving chemotherapy. You may be admitted to the hospital to receive CAR T-cell therapy.
After receiving CAR T-cell therapy, you will be closely monitored by your care team at MedStar Georgetown. Depending on the type of CAR T-cell therapy given, some patients may remain in the hospital for two weeks after their CAR T-cell infusion.
The biggest side effects of CAR T-cell therapy include the development of cytokine release syndrome (CRS) and neurotoxicities. CRS syndrome means that your body is setting up an immune response and substances called cytokines are released. Signs of CRS include lowered blood pressure, fever, rash, chills, and abnormal laboratory values. If patients experience severe side effects after CAR T-cell therapy, they may require care in an intensive care unit (ICU).
Neurotoxicity signs include dizziness, forgetfulness, and seizures. Some patients may experience difficulty in speaking or writing. Your doctor and clinical team will monitor you carefully for abnormal symptoms and signs of neurotoxicity.
After CAR T-cell treatment
Treatment does not end after CAR T-cell therapy. You will require monitoring over a period of years to ensure that your cancer does not come back.
You will still need to see your primary oncologist for regular monitoring and check-ups. You will continue to see your Cellular Immunotherapy care team at MedStar Georgetown.
Videos
Awards and recognitions

Our program at MedStar Georgetown University Hospital has been awarded FACT Accreditation—a recognition that indicates our program has met the most rigorous quality standards in every aspect of stem cell therapy. Home to the nations’ largest stem cell collection facility, is the region’s only adult, Foundation for the Accreditation of Cellular Therapy (FACT)-accredited program for autologous transplant, allogeneic transplant, and cellular immunotherapy. In addition, MedStar Georgetown is the only hospital in the area authorized to use six available FDA-approved CAR T-cell therapy drugs to treat blood cancers.
Patient stories
Frequently asked questions
-
What are the benefits of CAR T-cell therapy?
The primary benefit of this type of therapy is that it provides an additional treatment option for patients whose disease hasn’t responded to other treatments. As a result, patients usually have longer times in remission after CAR T-cell therapy and in some cases, the treatment can cure certain types of blood cancer.
-
How long does it take to start CAR T-cell therapy?
From your first appointment, the length of time until your therapy begins will vary based on several factors, including the manufacturing time specific to the product that will be used in your treatment. On average, most patients should expect the steps of the process to take several weeks to several months. Once we determine that you are eligible for this type of therapy, your care team will help you thoroughly understand each step of the process and what to expect, including timing.
-
What conditions are treated with this therapy?
Chimeric antigen receptor therapy, or CAR T-cell therapy is FDA-approved to treat multiple myeloma and certain kinds of lymphomas, including the following subtypes:
- High-grade B cell lymphoma
- Follicular lymphoma
- Primary mediastinal large B cell lymphoma
In many cases, this type of therapy is an option after other treatments have failed. Talk to your doctor about the best treatment options for you, including your eligibility for CAR T-cell therapy.
-
What are some common side effects of CAR T-cell therapy?
The biggest side effects of CAR T-cell therapy include the development of cytokine release syndrome (CRS) and neurotoxicities. CRS syndrome means that your body is setting up an immune response and substances called cytokines are released. Signs of CRS include lowered blood pressure, fever, rash, chills, and abnormal laboratory values. If patients experience severe side effects after CAR T-cell therapy, they may require care in an intensive care unit (ICU).
Neurotoxicity signs can range from mild, such as tremors or difficulty remembering certain words, to severe, such as seizures. Your doctor and clinical team will monitor you carefully for abnormal symptoms and signs of neurotoxicity.
After your treatment, your care team will continue to monitor you closely and help manage any potential side effects, such as:
- Fatigue
- Low blood counts that may require blood transfusions
- A weakened immune system
Our providers
Location: Change location Enter your location
-
Sophia Chang, AGACNP MSN
Hematology and Oncology
-
Molly Ann Lloyd, AGACNP-BC MSN
Hematology and Oncology
-
Anna Mantecon Garcia, FNP MSN
Hematology and Oncology & Blood and Marrow Transplantation
-
Kamil Rechache, MD
Hematopoietic Cell Transplantation and Cellular Therapy, Hematology and Oncology, Blood and Marrow Transplantation & Internal Medicine
-
Jennifer Ann Kanakry, MD
Hematopoietic Cell Transplantation and Cellular Therapy, Blood and Marrow Transplantation, Internal Medicine & Hematology and Oncology
Our location
Distance from Change locationEnter your location
MedStar Health: Stem Cell Transplant and Cellular Immunotherapy Program at MedStar Georgetown University Hospital
3800 Reservoir Rd., NW 2 East Main Building Washington, DC 20007











