MedStar Health is First in Maryland to Replace Mitral Valve Via Tendyne Transcatheter
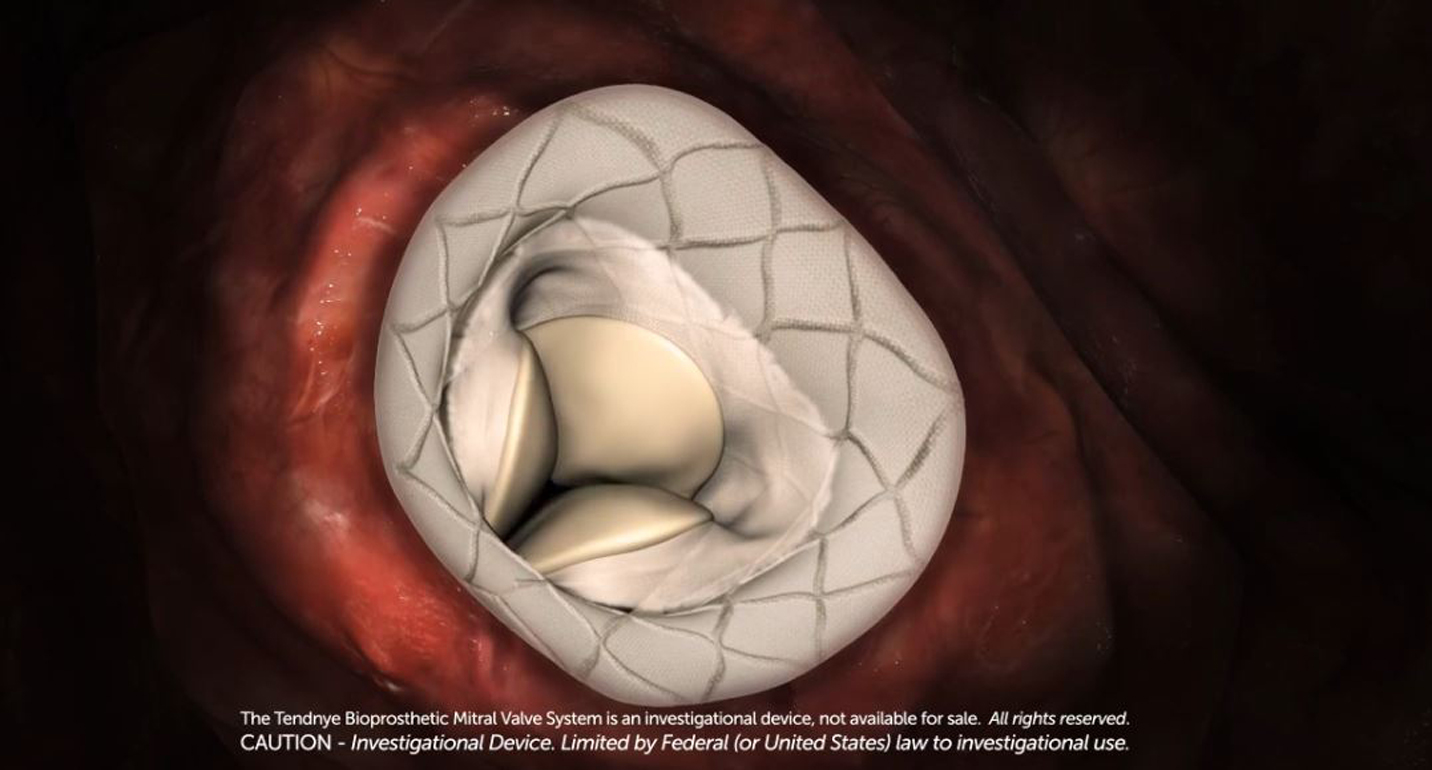
BALTIMORE—Drs. Brian Bethea, John Wang, Nauman Siddiqi and Antony Kaliyadan of the MedStar Heart and Vascular Institute (MHVI) at MedStar Union Memorial Hospital, successfully implanted the state’s first Tendyne transcather mitral valve replacement (TMVR) system November 14, as part of a national clinical trial testing the safety and efficacy of the implant as an option to open-heart surgery. Dr. Bethea is the MedStar principal investigator on the trial.
The patient, an 86-year-old Pikesville woman, was enrolled in the trial because of mitral regurgitation, (MR) a debilitating, progressive and life-threatening condition in which the heart's mitral valve does not close completely, causing blood to flow backward and leak into the left atrium of the heart.
The backward flow causes severe fatigue, shortness of breath, lightheadedness and a rapid, fluttering heartbeat. Severe cases can cause fluid to build up in the lungs, strain the heart and lead to heart failure. Patients with MR are also at risk for blood clots and stroke.
Four million Americans are affected by mitral regurgitation with only about 20 percent treatable by open heart surgery.
“The patient in this case really had limited treatment options because of the severity of her valvular disease her age and other factors,” said Dr. Bethea, Vice Chief of Cardiac Surgery at MedStar Union Memorial. “This is truly a groundbreaking advancement that is potentially lifesaving for patients who would not be able to undergo the risks of open-heart surgery. It will dramatically improve their quality of life.”
The device is a tri-leaflet, bioprosthetic valve available in multiple sizes, that once inserted through a small puncture in the side of the chest, and into a tiny incision at the tip of the heart is positioned inside the patient’s own mitral valve. Using a unique tether mechanism, the valve position is stabilized when the apical pad is applied directly to the heart.
The Tendyne mitral valve system is the first and only mitral valve replacement device that can be repositioned and fully retrieved, ensuring precise placement during implantation, which could improve patient outcomes.
The clinical trial, called SUMMIT, is expected to enroll 1,010 patients at 80 sites across the U.S., Canada and the European Union.
Tendyne Mitral Valve System is under investigation by FDA and is not commercially available.
Please click here more information.
#
About MedStar Health
MedStar Health is a not-for-profit health system dedicated to caring for people in Maryland and the Washington, D.C., region, while advancing the practice of medicine through education, innovation and research. MedStar’s 30,000 associates, 6,000 affiliated physicians, 10 hospitals, ambulatory care and urgent care centers, and the MedStar Health Research Institute are recognized regionally and nationally for excellence in medical care. As the medical education and clinical partner of Georgetown University, MedStar trains more than 1,100 medical residents annually. MedStar Health’s patient-first philosophy combines care, compassion and clinical excellence with an emphasis on customer service. For more information, visit MedStarHealth.org.
Categories
Media Contact
Debra Schindler
Regional Director, Media & Public Relations, MedStar Health Baltimore
410-274-1260debra.schindler@medstar.net









