Find a clinical trial
Please use one or more of the field below to begin your search.
All clinical trials
-
Protocol number: 00008478
Pilot Randomized Controlled Trial of Use of a Website about Radioactive Iodine Symptom Management in Patients with Thyroid Cancer
-
Protocol number: 022-REDUCLN-002
Efficacy of the COronary SInus Reducer in Patients with Refractory Angina II (COSIRA-II)
-
Protocol number: 1305-0031
An open-label extension trial of the long-term safety and efficacy of BI 1015550 taken orally in patients with idiopathic pulmonary fibrosis (IPF) and progressive pulmonary fibrosis (PPF) (FIBRONEER-ON)
-
Protocol number: 2013-0496
A cross-over pilot study of the effect of amiloride compared to triamterene on proteinuria in patients with proteinuric kidney disease
-
Protocol number: 2014-0755
Identifying children with diabetes type 1 at high risk for cardiovascular disease
-
Protocol number: 2017-0478
STEPs to Blood Pressure Reduction. A Randomized Clinical Trial of the Stroke Transitions, Education, and Prevention Clinic Versus Usual Care for Post-Stroke Blood Pressure Reduction
-
Protocol number: 2017-0700-NCT03685461
Intra- and Post-Operative Measures of Auditory Function
-
Protocol number: 20172841-PREDICT-II
A Prospective Registry Study to Evaluate the Effect of the DCISionRT Test on Treatment Decisions in Patients with DCIS Following Breast Conserving Therapy
-
Protocol number: 2018-0439
A Randomized Trial of Proactive Outreach and Streamlined Genetic Education in BRCA Families
-
Protocol number: 2018-242
Outcomes Evaluation of the Medial Femoral Trochlea Flap for Reconstruction of the Scaphoid and Lunate
-
Protocol number: 20180244-OCEANA
A Double-blind, Randomized, Placebo-controlled, Multicenter Study Assessing the Impact of Olpasiran on Major Cardiovascular Events in Patients with Atherosclerotic Cardiovascular Disease and Elevated Lipoprotein (a)
-
Protocol number: 2022-523-GLOB1
A MULTICENTER, PHASE 1B STUDY TO EVALUATE THE SAFETY, TOLERABILITY, PHARMACOKINETICS, AND PRELIMINARY EFFICACY OF HMPL-523, A SYK INHIBITOR, IN ADULT SUBJECTS WITH IMMUNE THROMBOCYTOPENIA
-
Protocol number: 20297A
placebo-controlled, dose-finding trial of Lu AG09222 for the prevention of migraine in participants with unsuccessful prior preventive treatments
-
Protocol number: 209639-VH3810109
A Phase 2b Multicenter, Randomized, Open-Label Study Comparing the Efficacy, Safety, PK, and Tolerability of VH3810109, Administered Either Intravenously Or As A Subcutaneous Infusion with rHuPH20, in Combination with CAB LA to Standard of Care in Virologically Suppressed Adults Living with HIV
-
Protocol number: 21-02-348-NCT05155501
Pelvic fascia spARing radical prostatectomy TrIAL (PARTIAL)
-
Protocol number: 218224
A Phase 2/3, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of belimumab administered subcutaneously in adults with systemic sclerosis-associated interstitial lung disease (SSc-ILD)
-
Protocol number: 221AD304
A Study to Evaluate Safety and Tolerability of Aducanumab in Participants With Alzheimer's Disease Who Had Previously Participated in the Aducanumab Studies 221AD103, 221AD301, 221AD302 and 221AD205 (EMBARK)
-
Protocol number: 230LE303
A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study to Evaluate the Efficacy and Safety of BIIB059 in Adult Participants with Active Systemic Lupus Erythematosus Receiving Background Nonbiologic Lupus Standard of Care
-
Protocol number: 230LE306
A Multicenter, Randomized, Dose-Blind, Phase 3 Long-Term Extension Study to Evaluate Continuous Safety and Efficacy of Litilfilimab (BIIB059) in Adult Participants with Active Systemic Lupus Erythematosus
-
Protocol number: 3082-CL-0101-NCT05382559
A Phase 1 Study of ASP3082 in Participants With Previously Treated Locally Advanced or Metastatic Solid Tumor Malignancies With KRAS G12D Mutation
-
Protocol number: 311-MOMENTUM-GHAZI
Study of the Prevalence of Endogenous Hypercortisolism in Patients with Resistant Hypertension (MOMENTUM)
-
Protocol number: 311-MOMENTUM-PARK
Study of the Prevalence of Endogenous Hypercortisolism in Patients with Resistant Hypertension (MOMENTUM)
-
Protocol number: 5063
: Role of a CCK receptor antagonist proglumide in management of chronic pancreatitis
-
Protocol number: 5500042287
Assessing and validating the psychometric properties of the CRISTAL PRO through an online registry
-
Protocol number: 61186372COR3001
A Randomized, Open-label Phase 3 Study of Amivantamab and mFOLFOX6 or FOLFIRI Versus Cetuximab and mFOLFOX6 or FOLFIRI as First-line Treatment in Participants With KRAS/NRAS and BRAF Wild-type Unresectable or Metastatic Left-sided Colorectal Cancer
-
Protocol number: 61186372COR3001-NCT06662786
A Randomized, Open-label Phase 3 Study of Amivantamab and mFOLFOX6 or FOLFIRI Versus Cetuximab and mFOLFOX6 or FOLFIRI as First-line Treatment in Participants With KRAS/NRAS and BRAF Wild-type Unresectable or Metastatic Left-sided Colorectal Cancer
-
Protocol number: 61186372COR3002
A Randomized, Open-label Phase 3 Study of Amivantamab + FOLFIRI Versus Cetuximab/Bevacizumab + FOLFIRI in Participants With KRAS/NRAS and BRAF Wildtype Recurrent, Unresectable or Metastatic Colorectal Cancer Who Have Received Prior Chemotherapy
-
Protocol number: 61186372NSC2012-NCT06667076
A Phase 2b, Open-Label, Two-cohort Study of Subcutaneous Amivantamab in Combination With Lazertinib as First-Line Treatment, or Subcutaneous Amivantamab in Combination With Platinum-Based Chemotherapy as Second-line Treatment, for Common EGFR-Mutated Locally Advanced or Metastatic NSCLC
-
Protocol number: 63733657ALZ2002
A Randomized, Double-blind, Placebo-controlled, Parallel-group, Multicenter Study to Assess the Efficacy and Safety of JNJ-63733657, an Anti-tau Monoclonal Antibody, in Participants With Early Alzheimer's Disease
-
Protocol number: 64407564MMY3002-NCT05455320
A Phase 3 Randomized Study Comparing Talquetamab SC in Combination With Daratumumab SC and Pomalidomide (Tal-DP) or Talquetamab SC in Combination With Daratumumab SC (Tal-D) Versus Daratumumab SC, Pomalidomide and Dexamethasone (DPd), in Participants With Relapsed or Refractory Multiple Myeloma who Have Received at Least 1 Prior Line of Therapy
-
Protocol number: 68284528MMY4006-NCT05346835
Intermediate-Size Population Expanded Access Program (EAP) for Ciltacabtagene autoleucel (cilta-cel) Out-of-Specification (OOS) in patients with Multiple Myeloma
-
Protocol number: 718-CIH-201
A Randomized, Placebo-Controlled, Double-Blind Study to Evaluate the Effect of SAGE-718 on Cognitive Function in Participants with Huntington s Disease
-
Protocol number: 718-CIH-202
A 28-Day Randomized, Placebo-Controlled, Double-Blind, Parallel Groups and Normative Comparison Study to Evaluate the Effect of SAGE-718 on Functioning Capacity in Participants with Huntington's Disease
-
Protocol number: 718-CIH-301
PHASE 3 OPEN-LABEL SAFETY STUDY OF SAGE-718 IN PARTICIPANTS WITH HUNTINGTON'S DISEASE
-
Protocol number: 849-007-KRYSTAL-7
A Phase 2 Trial of Adagrasib Monotherapy and in Combination with Pembrolizumab and a Phase 3 Trial of Adagrasib in Combination with Pembrolizumab versus Pembrolizumab in Patients with Advanced Non-Small Cell Lung Cancer with KRAS G12C Mutation
-
Protocol number: A012103
De-Escalation of Therapy in Early-Stage TNBC Patients Who Achieve pCR After Neoadjuvant Chemotherapy with Checkpoint Inhibitor Therapy"
-
Protocol number: A012303-NCT06876714
ShortStop-HER2: Shortened Duration of Adjuvant Therapy in Patients with Early-Stage HER2+ Breast Cancer Who Achieve pCR After Neoadjuvant Chemotherapy with HER2 Blockade
-
Protocol number: A021901-NCT04665739
Randomized Phase II Trial of Lutetium Lu 177 Dotatate Versus Everolimus in Somatostatin Receptor Positive Bronchial Neuroendocrine Tumors
-
Protocol number: A022102-NCT05677490
Randomized Phase III Trial of mFOLFIRINOX vs. FOLFOX With Nivolumab for First-Line Treatment of Metastatic HER2- Gastroesophageal Adenocarcinoma
-
Protocol number: A032103-NCT05987241
MODERN: An Integrated Phase 2/3 and Phase 3 Trial of MRD-Based Optimization of ADjuvant ThErapy in URothelial CaNcer
-
Protocol number: A032201-STRIKE
A032201: Short TeRm Intensified Pembrolizumab (KEytruda) and Tivozanib for High-Risk Renal Cell Carcinoma - STRIKE
-
Protocol number: A032302-ASPIRE-NCT06931340
A032302: Docetaxel Addition in Metastatic Castrate-Sensitive Prostate Cancer (ASPIRE)
-
Protocol number: A041703-NCT03739814
A Phase II Study of Inotuzumab Ozogamicin Followed by Blinatumomab for Ph-Negative CD22-Positive B-Lineage Acute Lymphoblastic Leukemia in Newly Diagnosed Older Adults or Adults With Relapsed or Refractory Disease
-
Protocol number: A051902-NCT04803201
A RANDOMIZED PHASE II STUDY OF CHO(E)P VS CC-486-CHO(E)P VS DUVELISIB-CHO(E)P IN PREVIOUSLY UNTREATED CD30 NEGATIVE PERIPHERAL T-CELL LYMPHOMAS
-
Protocol number: A081801-NCT04267848
Testing the Addition of a Type of Drug Called Immunotherapy to the Usual Chemotherapy Treatment for Non-small Cell Lung Cancer, ALCHEMIST Chemo-IO Study
-
Protocol number: A092107
A RANDOMIZED PHASE 2 TRIAL WITH A SAFETY LEAD-IN TO EVALUATE PALBOCICLIB VERSUS PALBOCICLIB AND CEMIPLIMAB FOR THE TREATMENT OF ADVANCED DEDIFFERENTIATED LIPOSARCOMA
-
Protocol number: A211801-BRCA-P-NCT04711109
A Randomized, Double-Blind, Placebo-Controlled, Multi-Center, International Phase 3 Study to determine the Preventive Effect of Denosumab on Breast Cancer in Women carrying a BRCA1 Germline Mutation
-
Protocol number: A212102
Collecting Blood Samples From Patients With and Without Cancer to Evaluate Tests for Early Cancer Detection
-
Protocol number: A212102-MULTICANCER-NCT0533406
Blinded Reference Set For Multicancer Early Detection Blood Tests
-
Protocol number: A22-301
A Single-arm, Multicenter Study to Assess the Efficacy, Safety, and Tolerability of Ropeginterferon alfa-2b-njft (P1101) in Adult Patients with Essential Thrombocythemia
-
Protocol number: A23-302
A Randomized, Double-Blind, Placebo-Controlled Multicenter Phase 3 Clinical Study to Assess Efficacy and Safety of Ropeginterferon alfa-2b (P1101) in Adult Patients with Pre-fibrotic/Early Primary Myelofibrosis or Overt Primary Myelofibrosis at Low or Intermediate-1 Risk According to DIPSS Plus (HOPE-PMF): The Core Study and Its Extension Study
-
Protocol number: AALL1732-NCT03959085
A Phase 3 Randomized Trial of Inotuzumab Ozogamicin (IND#:133494, NSC#: 772518) for Newly Diagnosed High-Risk B-ALL; Risk-Adapted Post-Induction Therapy for High-Risk B-ALL, Mixed Phenotype Acute Leukemia, and Disseminated B-LLy
-
Protocol number: AALL2131-NCT06124157
An International Pilot Study of Chemotherapy and Tyrosine Kinase Inhibitors with Blinatumomab in Patients with Newly-Diagnosed Philadelphia Chromosome-Positive or ABL-Class Philadelphia Chromosome-Like B-Cell Acute Lymphoblastic Leukemia
-
Protocol number: AAML1831-NCT04293562
A Phase 3 Randomized Trial for Patients with de novo AML Comparing Standard Therapy Including Gemtuzumab Ozogamicin (GO) to CPX-351 with GO, and the Addition of the FLT3 Inhibitor Gilteritinib for Patients with FLT3 Mutations.
-
Protocol number: ABBOTTIVLIDE
Abbott Coronary Intravascular Lithotripsy (IVL) IDE study
-
Protocol number: ABBOTTTEAMHF
Trial to Evaluate Safety And Effectiveness of Mechanical Circulatory Support in Patients with Advancing Heart Failure
-
Protocol number: ABBVP22-487
A Global Real World Evidence Study on the long-term effectiveness of ABBV-951 in Advanced Parkinson ́s Disease in routine clinical practice (ROSSINI: Real-world Outcomes with continuouS Subcutaneous levodopa INfusIon)
-
Protocol number: ABLE-22-NCT06545955
A Phase 2, Randomised, Multi-center, Open Label Trial to Evaluate the Safety and Efficacy of Intravesical Nadofaragene Firadenovec Alone or in Combination With Chemotherapy or Immunotherapy in Participants With High-grade Bacillus Calmette-Guerin Therapy (BCG) Unresponsive Non-muscle Invasive Bladder Cancer (NMIBC)
-
Protocol number: ACCLAIM-LPA
A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Effect of Lepodisiran on the Reduction of Major Adverse Cardiovascular Events in Adults with Elevated Lipoprotein(a) who have Established Atherosclerotic Cardiovascular Disease or Are at Risk for a First Cardiovascular Event1; ACCLAIM-Lp(a)
-
Protocol number: ACT-RESEARCH
Assessing the Acceptability of an Acceptance and Commitment Therapy (ACT). Intervention for Supporting Adjustment to Appearance Changes After Burn Injury
-
Protocol number: ACT17209
A multicenter, open-label, Phase IIb study to evaluate the efficacy, safety and pharmacokinetics of rilzabrutinib in patients with warm autoimmune hemolytic anemia
-
Protocol number: ACTNOW
Pragmatic, Randomized, Blinded Trial to Shorten Pharmacologic Treatment of Newborns with Neonatal Opioid Withdrawal Syndrome (NOWS)
-
Protocol number: ADMIRE-BWI201910
Assessment of Safety and Effectiveness in Treatment Management of Atrial Fibrillation with the BWI IRE Ablation System
-
Protocol number: ADNI3-NCT02854033
Alzheimers Disease Neuroimaging Initiative 3 (ADNI3)
-
Protocol number: ADVANCE-STUDY
EndurAnt Stent Graft system vs ExcluDer endoprothesis: a global, prospectiVe, rANdomized Clinical trial in sac rEgression (ADVANCE Study)
-
Protocol number: ADVENTLTO
ADVENT Trial Long Term Outcomes Evaluating Atrial Fibrillation Progression
-
Protocol number: ADVENTPAS
Prospective, Multi-site Safety and Effectiveness Post-Approval Study of FARAPULSE Pulsed Field Ablation in Paroxysmal Atrial Fibrillation
-
Protocol number: AFFINITY-NCT03222973
A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study in Subjects With Relapsing Multiple Sclerosis to Evaluate the Efficacy and Safety of BIIB033 as an Add-On Therapy to Anti-Inflammatory Disease-Modifying Therapies
-
Protocol number: AG348-C-020
A Phase 2/3, Double-Blind, Randomized, Placebo-Controlled, Multicenter Study to Evaluate the Efficacy and Safety of Mitapivat in Subjects With Sickle Cell Disease
-
Protocol number: AG946-C-003
A Phase 2, Double-blind, Randomized, Placebo-Controlled, Multicenter, Dose-Finding, Efficacy, and Safety Study of Tebapivat in Participants With Sickle Cell Disease
-
Protocol number: AGCT1531-NCT03067181
A Phase 3 Study of Active Surveillance for Low Risk and a Randomized Trial of Carboplatin vs. Cisplatin for Standard Risk Pediatric and Adult Patients With Germ Cell Tumors
-
Protocol number: AGCT1532-NCT02582697
Phase 3 Accelerated BEP: A Randomised Phase 3 Trial of Accelerated Versus Standard BEP Chemotherapy for Patients With Intermediate and Poor-risk Metastatic Germ Cell Tumours
-
Protocol number: AHAEMI
EMICIZUMAB IN PATIENTS WITH ACQUIRED HEMOPHILIA A: MULTICENTER, SINGLE- ARM, OPEN-LABEL CLINICAL TRIAL
-
Protocol number: AHEAD3-45
AHEAD 3-45 Study: A Placebo-Controlled, Double-Blind, Parallel-Treatment Arm, 216 Week Study to Evaluate Efficacy and Safety of Treatment With BAN2401 in Subjects With Preclinical Alzheimer s Disease and Elevated Amyloid (A45 Trial) and in Subjects With Early Preclinical Alzheimer s Disease and Intermediate Amyloid (A3 Trial)
-
Protocol number: AHOD2131-NCT05675410
A Randomized Phase 3 Interim Response Adapted Trial Comparing Standard Therapy With Immuno-oncology Therapy for Children and Adults With Newly Diagnosed Stage I and II Classic Hodgkin Lymphoma
-
Protocol number: AIBERRY-STUDY00003473
Developing an A.I. Platform to Detect Depression Predict depression at higher degree of accuracy Implement the Aiberry platform in a live environment
-
Protocol number: AIS-D04
A PHASE 1B, OPEN-LABEL STUDY TO ASSESS THE SAFETY, EFFICACY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF ALPN-303 IN SUBJECTS WITH AUTOIMMUNE CYTOPENIAS (RUBY-4)
-
Protocol number: AK-US-001-0105
PHASE 3, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDY EVALUATING THE SAFETY AND EFFICACY OF EFRUXIFERMIN IN SUBJECTS WITH NON-CIRRHOTIC NONALCOHOLIC STEATOHEPATITIS (NASH)/METABOLIC DYSFUNCTION-ASSOCIATED STEATOHEPATITIS (MASH) AND FIBROSIS
-
Protocol number: AL002-2
A PHASE 2 RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, MULTICENTER STUDY TO EVALUATE THE EFFICACY AND SAFETY OF AL002 IN PARTICIPANTS WITH EARLY ALZHEIMER S DISEASE
-
Protocol number: AL002-LTE
A MULTICENTER, LONG-TERM EXTENSION STUDY TO EVALUATE THE SAFETY, TOLERABILITY, AND EFFICACY OF AL002 IN PARTICIPANTS WITH ALZHEIMER’S DISEASE
-
Protocol number: ALIGN-AR-NCT04415047
A Study to Assess Safety and Probable Benefit of the Transfemoral JenaValve Pericardial TAVR System in the Treatment of High Surgical Risk Patients with Symptomatic, Severe Aortic Regurgitation (AR)
-
Protocol number: ALL-RISE/NCT05893498
Advancing Cath Lab Results with FFRangio® Coronary Physiology Assessment: The ALL-RISE Study
-
Protocol number: ALLIANCE-AVIV
Safety and Effectiveness of Balloon-Expandable Bioprosthetic SAPIEN X4 Transcatheter Heart Valve in Failing Aortic Surgical Bioprosthetic Valves
-
Protocol number: ALLIANCE-NCT05172960
Safety and Effectiveness of Balloon-Expandable Bioprosthetic SAPIEN X4 Transcatheter Heart Valve
-
Protocol number: ALLO-316-101-NCT04696731
A PHASE 1 MULTICENTER STUDY EVALUATING THE SAFETY AND EFFICACY OF ALLO-316 FOLLOWING ALLO-647 CONTAINING CONDITIONING REGIMEN IN SUBJECTS WITH ADVANCED OR METASTATIC CLEAR CELL RENAL CELL CARCINOMA (ccRCC)
-
Protocol number: ALLO-501A-202-NCT06500273
A RANDOMIZED, OPEN-LABEL STUDY EVALUATING THE EFFICACY AND SAFETY OF CEMACABTAGENE ANSEGEDLEUCEL IN PARTICIPANTS WITH MINIMAL RESIDUAL DISEASE AFTER RESPONSE TO FIRST LINE THERAPY FOR LARGE B-CELL LYMPHOMA (ALPHA3)
-
Protocol number: ALN-TTRSC02-007-HELIOS-B-EXTEN
An Open-Label Extension Study to Assess the Safety and Efficacy of Vutrisiran in Patients with Transthyretin Amyloidosis with Cardiomyopathy (ATTR Amyloidosis with Cardiomyopathy)
-
Protocol number: ALTE1631-NCT03223753
A Randomized Web-based Physical Activity Intervention among Children and Adolescents with Cancer
-
Protocol number: ALX-NMO-501
A Long-Term, Prospective, Observational, Registry of Patients with Anti-Aquaporin 4 Antibody-Positive (AQP4+) Neuromyelitis Optica Spectrum Disorder (NMOSD) Treated with Alexion Complement Component 5 (C5) Inhibitor Therapies (ALXN-C5IT)
-
Protocol number: ALXN-MG-501
Long-Term, Observational, Global Registry of Patients With Generalized Myasthenia Gravis Who Have Received Treatment With Complement C5 Inhibition Therapies
-
Protocol number: ALXN2220-ATTRCM-301/NCT0618393
DepleTTR-CM A Phase 3, Randomized, Double-blind, Placebo-controlled, Multicenter Study to Evaluate the Efficacy and Safety of Amyloid Depleter ALXN2220 in Adult Participants with Transthyretin Amyloid Cardiomyopathy (ATTR-CM)
-
Protocol number: AMBITION
Randomized Controlled Trial of the Auryon Atherectomy System Used in Combination with Standard Balloon Angioplasty Versus Standard Balloon Angioplasty Alone Treating Infrapopliteal Lesions in Subjects with Critical Limb Ischemia: the Auryon AMBITION BTK RCT / Registry
-
Protocol number: AMBULATEEXPAND
A MULTI-CENTER, PROSPECTIVE, SINGLE ARM, PIVOTAL TRIAL TO EVALUATE THE SAFETY AND EFFICACY OF THE VASCADE MVP® XL VENOUS VASCULAR CLOSURE SYSTEM (VVCS) FOR THE MANAGEMENT OF THE FEMORAL VENOTOMY AFTER CATHETER-BASED INTERVENTIONS UTILIZING 16-17F OUTER DIAMETER (OD) SHEATHS
-
Protocol number: AMG-193-20230153-NCT05975073
A Phase 2 Study Evaluating the Efficacy, Safety, Tolerability, and Pharmacokinetics of AMG 193 in Subjects with Methylthioadenosine Phosphorylase (MTAP)-deleted Previously Treated Advanced Non Small Cell Lung Cancer (NSCLC)
-
Protocol number: AMGEN-20230222
A Double-blind, Randomized, Placebo-controlled, Multicenter Study Assessing Olpasiran Use to Prevent First Major Cardiovascular Events in Participants with Elevated Lipoprotein(a)
-
Protocol number: AML-PROSPECTIVE-STUDY00000298
A Pilot Study to Evaluate Physical Function and Fatigue in Patients with Acute Myeloid Leukemia Single Center Prospective Cohort Study
-
Protocol number: AML-RETROSPECTIVE
A center-based chart review study to assess treatment outcomes of venetoclax for the treatment of acute myeloid leukemia (AML)
-
Protocol number: AMPLATZER-PFO-NCT03309332
AMPLATZER PFO Occluder Post Approval Study (PFO PAS)
-
Protocol number: AMPRELOXETINE-0197-CYPRESS
A Phase 3, Multi-center, Randomized Withdrawal and Long-Term Extension Study of Ampreloxetine for the Treatment of Symptomatic Neurogenic Orthostatic Hypotension in Participants with Multiple System Atrophy
-
Protocol number: AMS05
Randomized, Blinded Discontinuation Trial of Ocrelizumab in Early Relapsing Multiple Sclerosis
-
Protocol number: ANHL1931-NCT04759586
Nivolumab in Combination With Chemo-Immunotherapy for the Treatment of Newly Diagnosed Primary Mediastinal B-Cell Lymphoma
-
Protocol number: ANTHOS-NCT05712200
A Phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of abelacimab in high-risk patients with Atrial fibrillation who have been deemed unsuitable for oral anticoagulation
-
Protocol number: AP-PA02-201
A Phase 2, Multi-Center, Double-Blind, Randomized, Placebo Controlled Study to Evaluate the Safety, Phage Kinetics, and Efficacy of Inhaled AP-PA02 Multi-Phage Therapeutic in Subjects with Non-Cystic Fibrosis Bronchiectasis and Chronic Pulmonary Pseudomonas aeruginosa Infection
-
Protocol number: APAL2020SC-NCT04726241
Pediatric Acute Leukemia (PedAL) Screening Trial - Developing New Therapies for Relapsed Leukemias
-
Protocol number: APEC14B1
APEC14B1: Project:EveryChild A Registry, Eligibility Screening, Biology and Outcome Study
-
Protocol number: APEC1621SC-NCT03155620
NCI-COG Pediatric MATCH (Molecular Analysis for Therapy Choice) Screening Protocol
-
Protocol number: APOL-1
Integrating a Culturally Competent APOL1 Genetic Testing Program into Living Donor Evaluation
-
Protocol number: APOLLO-NCT03242642
Transcatheter Mitral Valve Replacement with the Medtronic Intrepid TMVR System in patients with severe symptomatic mitral regurgitation APOLLO Trial
-
Protocol number: APPROVE
A randomized controlled trial evaluating the efficacy and safety of a prescription digital therapeutic for the treatment of overactive bladder in women
-
Protocol number: APT-DFI
A Phase 2b Randomized, Parallel Group, Double-Blind, Placebo-Controlled, Repeat Dose, MultiSite Study Investigating the Safety, Tolerability, and Efficacy of Personalized Phage Treatment and Standard of Care Antimicrobials for Subjects with Diabetic Foot Osteomyelitis due to S. aureus
-
Protocol number: AREN1921-NCT04322318
Treatment of Newly Diagnosed Diffuse Anaplastic Wilms Tumors(DAWT)and Relapsed Favorable Histology Wilms Tumors(FHWT)
-
Protocol number: ARGONCLEAN-PE
CLEAN-PE: A Prospective, Multicenter Study to Evaluate the Safety and Efficacy of an Aspiration Thrombectomy System in Acute Pulmonary Embolism
-
Protocol number: ARGX-113-2007-NCT05523167
A Phase 2/3, Randomized, Double-Blinded, Placebo-Controlled, Parallel-Group, 2-Arm, Multicenter, Operationally Seamless Study to Evaluate the Efficacy, Safety, Tolerability, Pharmacodynamics, Pharmacokinetics, and Immunogenicity of Efgartigimod PH20 SC in Participants Aged 18 Years and Older With Active Idiopathic Inflammatory Myopathy
-
Protocol number: ARGX-113-2402
A Phase 3, Multicenter, Randomized, Double-Blinded, Placebo- Controlled, Parallel-Arm Study Followed by an Open-Label Arm to Evaluate the Efficacy and Safety of Efgartigimod IV in Adult Participants With Primary Immune Thrombocytopenia
-
Protocol number: ARGX-113-2406
A Phase 4, Open-Label, Single-Group, Multicenter Study in Adult Participants With Chronic Inflammatory Demyelinating Polyneuropathy Who Transition From Treatment With Intravenous Immunoglobulin to Efgartigimod PH20 SC
-
Protocol number: ARGX-117-2202
A Multicenter Prospective Longitudinal Study of Clinical Outcomes, Disease Course, Health-Related Quality of Life, and Health Care Resource Utilization in Adult Patients With Multifocal Motor Neuropathy
-
Protocol number: ARGX-117-2302
A Phase 3, Randomized, Double-Blinded, Double-Dummy Study Evaluating the Efficacy and Safety of Empasiprubart Versus Intravenous Immunoglobulin in Adults With Multifocal Motor Neuropathy
-
Protocol number: ARISEII-NCT05800743
Evaluation of the GORE® Ascending Stent Graft (ASG device) in the Treatment of Lesions of the Ascending Aorta
-
Protocol number: ARRIVAL-D5180C00047
A Multicentre, Randomised, Open-Label, Parallel-Group, Phase IIIb Study to Assess the Potential for Tezepelumab-treated Patients with Severe Asthma to Reduce Background Therapy While Sustaining Asthma Control and Clinical Remission (ARRIVAL)
-
Protocol number: ASCENDCSP
A Stylet-Driven ICD Lead Intended for Conduction System Pacing IDE Study
-
Protocol number: ASELL-SPECIAL-POPULATIONS
Clinical Validation of the CytoRADx System as a Biodosimeter for Special Human Populations
-
Protocol number: ASK-PD5-CS201
A Phase 2, Randomized, Double-blind, Sham Surgery-controlled Study of the Efficacy and Safety of Intraputaminal AAV2-GDNF in the Treatment of Adults with Moderate Stage Parkinson’s Disease
-
Protocol number: ASOLO-SSC-NCT06661473
An interventional study to explore the occurrence of surgical site complications in revision total hip or knee joint arthroplasty patients receiving closed incision negative pressure wound therapy.
-
Protocol number: ASPEN-INTERVIEW
Qualitative Interviews with Patients of the Brensocatib Post-Trial Access Program
-
Protocol number: ASPIRE-NCT03907046-GUMC
Anticoagulation in Intracerebral Hemorrhage (ICH) Survivors for Stroke Prevention and Recovery
-
Protocol number: AT-1501-K207
BESTOW: A Phase 2, Multicenter, Randomized, Open-Label Study to Evaluate the Safety and Efficacy of Tegoprubart in Patients Undergoing Kidney Transplantation
-
Protocol number: AT-1501-K209
A Phase 2, Multicenter, Open-Label Extension Study to Evaluate the Long-Term Safety and Efficacy of Tegoprubart in Kidney Transplant Recipients
-
Protocol number: ATA129-EBV-302-NCT03394365
Multicenter, Open Label, Phase 3 Study of Tabelecleucel for Solid Organ or Allogeneic Hematopoietic Cell Transplant Subjects With Epstein-Barr Virus-Associated Post-Transplant Lymphoproliferative Disease After Failure of Rituximab or Rituximab and Chemotherapy
-
Protocol number: ATN-106
A multicentre, prospective, open-label, uncontrolled Phase 3 study to assess the efficacy, safety and pharmacokinetics of Atenativ in patients with congenital antithrombin deficiency undergoing surgery or delivery
-
Protocol number: ATRI-011-C
ATRI Protocol Template v2.0 15 February 2023 1 Alzheimer’s Disease Neuroimaging Initiative 4 (ADNI4):
-
Protocol number: AVTX-009-HS-201
A Phase 2, Randomized, Double-blind, PlaceboÂcontrolled, Parallel-group Study to Evaluate the Efficacy and Safety of A VTX-009 in Patients with Moderate to Severe Hidradenitis Suppurativa (LOTUS)
-
Protocol number: AVZO-1418-1001-NCT07038343
A phase 1/2, first in human study to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and anti-tumor activity of AVZO-1418 as a single agent and in combination therapy in patients with locally advanced or metastatic solid tumors
-
Protocol number: AXONICS-105-0107
Single-Arm, Multi-Center, Prospective Study to Assess the Foramen FinderTM during Peripheral Nerve Evaluation (PNE)
-
Protocol number: B-64-NCT06401421
EXActDNA-003 / NSABP B-64: Breast Cancer Clinical Validation Study to Predict Recurrence of High-Risk Early Breast Cancer Treated With Neoadjuvant Therapy Using a Bespoke Circulating Tumor DNA Assay to Detect Molecular Residual Disease
-
Protocol number: B7471015
A PHASE 4 STUDY USING A TEST-NEGATIVE DESIGN TO EVALUATE THE EFFECTIVENESS OF A 20-VALENT PNEUMOCOCCAL CONJUGATE VACCINE AGAINST VACCINE-TYPE RADIOLOGICALLY-CONFIRMED COMMUNITYACQUIRED PNEUMONIA IN ADULTS </= to 65 YEARS OF AGE
-
Protocol number: B7981040
A PHASE 3 RANDOMIZED, DOUBLE-BLIND, 52-WEEK PLACEBOCONTROLLED, MULTI-CENTER STUDY INVESTIGATING THE EFFICACY, SAFETY, AND TOLERABILITY OF RITLECITINIB IN ADULT AND ADOLESCENT PARTICIPANTS WITH NON SEGMENTAL VITILIGO
-
Protocol number: BACKBEATUMH
BradycArdia paCemaKer with AV interval modulation for Blood prEssure treAtmenT (BACKBEAT Trial)
-
Protocol number: BAN2401-G000-301
A Placebo-Controlled, Double-Blind, Parallel-Group, 18-Month Study With an Open-Label Extension Phase to Confirm Safety and Efficacy of BAN2401 in Subjects With Early Alzheimer s Disease.
-
Protocol number: BAYER-FINEONE
A parallel-group, randomized, prospective, interventional, double-blind, multicenter global Phase 3 study to investigate the efficacy and safety of finerenone versus placebo, in addition to standard of care, in participants with chronic kidney disease and type 1 diabetes
-
Protocol number: BB2121-EAP-001-NCT04771078
EXPANDED ACCESS PROTOCOL (EAP) FOR SUBJECTS RECEIVING IDECABTAGENE VICLEUCEL THAT IS NONCONFORMING FOR COMMERCIAL RELEASE
-
Protocol number: BDTX-4933-101-NCT05786924
A Phase 1, Open-label Study of BDTX-4933 in Patients With BRAF and Other Select RAS/MAPK Mutation-Positive Neoplasms
-
Protocol number: BE1116
A Prospective, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Large Simple Trial Evaluating the Use of BE1116 (4-Factor Prothrombin Complex Concentrate [Kcentra® / Beriplex®]) to Improve Survival in Patients with Traumatic Injury and Acute Major Bleeding
-
Protocol number: BELIEVE
BELIEVE Trial: Bulking versus sling for treating stress urinary Incontinence at the time of Vaginal prolapse repair.
-
Protocol number: BEYONDD-001
Biomarker Evaluation in Young Onset Dementia from Diverse Populations (BEYONDD)
-
Protocol number: BFR-HITT-NCT06214494
Effects of Blood Flow Restricted Treadmill Training on Independently Ambulating Chronic Ischemic CVA Survivors: A Pilot Study
-
Protocol number: BGB-11417-201-NCT05471843
A Single-Arm, Open-Label, Multicenter Phase 2 Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Bcl-2 Inhibitor BGB-11417 in Patients With Relapsed or Refractory Mantle Cell Lymphoma
-
Protocol number: BGB-11417-203-NCT05952037
An Open-Label, Multicenter Phase 2 Study to Evaluate the Efficacy and Safety of the BCL2 Inhibitor Sonrotoclax (BGB-11417) as Monotherapy and in Combination with Zanubrutinib (BGB-3111) in Patients With Waldenström Macroglobulinemia
-
Protocol number: BGB-11417-301-NCT06073821
A Phase 3, Open-Label, Randomized Study of Sonrotoclax (BGB-11417) Plus Zanubrutinib (BGB-3111) Compared With Venetoclax Plus Obinutuzumab in Patients With Previously Untreated Chronic Lymphocytic Leukemia
-
Protocol number: BH-30643-01-NCT06706076
A Phase 1/2 Open-Label, Multicenter, First-in-Human Study of the Safety, Tolerability, Pharmacokinetics, and Antitumor Activity of BH-30643 in Adult Subjects With Locally Advanced or Metastatic NSCLC Harboring EGFR and/or HER2 Mutations (SOLARA)
-
Protocol number: BHV7000-303
A Phase 2/3 Multicenter, Randomized, Double-Blind, Placebo-Controlled, Study to Evaluate the Efficacy, Safety and Tolerability of BHV-7000 in Subjects with Refractory Focal Onset Epilepsy
-
Protocol number: BI-1479-0001-NCT04886804
An open label, Phase I dose escalation trial, with dose confirmation and expansion, of BI 1810631 as monotherapy in patients with advanced or metastatic solid tumors with HER2 aberrations
-
Protocol number: BILLIONTOONE
UNITY Patient Registry for the clinical validation of RhD Non-Invasive Prenatal Testing and Fetal Antigen Non-Invasive Prenatal Testing
-
Protocol number: BIO-CONDUCT-NCT05251363
BIOTRONIK Conduction System Pacing with the Solia Lead
-
Protocol number: BIOGEN-232SM303-ASCEND
A Phase 3b Study to Evaluate Higher Dose Nusinersen (BIIB058) in Patients With Spinal Muscular Atrophy Previously Treated With Risdiplam
-
Protocol number: BIOGEN-275AS101-ALSPIRE
A Phase 1/2 Multiple-Ascending-Dose Study With a Long-Term Open-Label Extension to Assess the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Effect on Disease Progression of BIIB105 Administered Intrathecally to Adults With Amyotrophic Lateral Sclerosis With or Without Poly-CAG Expansion in the Ataxin-2 Gene
-
Protocol number: BIPAVE-001-NCT06635785
Safety, Pharmacokinetics, and Efficacy of AI-081, a Bispecific Antibody for PD-1 And VEGF in Advanced Solid Tumors (BiPAVE-001)
-
Protocol number: BK-JM-201
A Randomized, Double-Blind, Placebo-Controlled, Two-Part Study in Parkinson’s Disease Patients With Dyskinesia to Assess the Efficacy and Safety/Tolerability of Fixed Dose Combinations of JM-010 and its Individual Components
-
Protocol number: BK-JM-201-NCT04377945
A Randomized, Double-Blind, Placebo-Controlled, Two-part Study in Parkinson’s Disease Patients With Dyskinesia to Assess the Efficacy and Safety/Tolerability of Fixed Dose Combinations of JM-010 and its Individual Components
-
Protocol number: BL-B01D1-LUNG-101-NCT05983432
A Phase 1 Study Evaluating the Safety, Tolerability, and Efficacy of BL-B01D1 in Subjects With Metastatic or Unresectable Non-Small Cell Lung Cancer
-
Protocol number: BMTCTN1903-NCT04975698
Administration of HIV-specific T cells to HIV+ Patients Receiving High Dose Chemotherapy Followed by Autologous Stem Cell Rescue -Auto-RESIST
-
Protocol number: BN42489
A PHASE II, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, DOSE-FINDING STUDY TO EVALUATE THE SAFETY, BIOMARKERS, AND EFFICACY OF TOMINERSEN IN INDIVIDUALS WITH PRODROMAL AND EARLY MANIFEST HUNTINGTON'S DISEASE
-
Protocol number: BNT323-01-GOG-3105
A Phase III, randomized, multi-site, open-label trial of BNT323/DB-1303 versus investigator s choice of chemotherapy in previously treated patients with HER2-expressing recurrent endometrial cancer
-
Protocol number: BO45230-NCT06534983
A RANDOMIZED PHASE II, DOUBLE-BLIND, MULTICENTER STUDY EVALUATING THE EFFICACY AND SAFETY OF AUTOGENE CEVUMERAN PLUS NIVOLUMAB VERSUS NIVOLUMAB AS ADJUVANT THERAPY IN PATIENTS WITH HIGH-RISK MUSCLEINVASIVE UROTHELIAL CARCINOMA
-
Protocol number: BOLT-NCT05003843
BOLT: A Prospective, Multicenter Study of Patients with Deep Vein Thrombosis to Evaluate the Safety and Efficacy of the Indigo® Aspiration System
-
Protocol number: BP-ARDS-P2-001
Phase 2 Clinical Platform Trial Investigating Multiple Therapeutic Options for the Treatment of Hospitalized Patients with Acute Respiratory Distress Syndrome (ARDS)
-
Protocol number: BP-LVAD
Blood Pressure and Outcomes in Contemporary Left Ventricular Assist Device Recipients
-
Protocol number: BQ7-EMAGINE-NCT06386874
the efficacy of a frequency-tuned electromagnetic field treatment in facilitating the recovery of subacute ischemic stroke patients - a pivotal study
-
Protocol number: BRIDGES-NCT05448560
BRidging Information Divides and Gaps for Equity in Survivorship (BRIDGES)
-
Protocol number: BRT-DA01-301
exPDite-2: A Phase 3 Study to Assess the Efficacy and Safety of Midbrain Dopaminergic Neuronal Cell Therapy (Bemdaneprocel) for Participants With Parkinson's Disease
-
Protocol number: BURN-RADIATION
A Systems Biology Approach to Radiation Biodosimetry and the Host- Environment Interaction: Applications to Mass Casualty Triage in the Polytrauma Patient.
-
Protocol number: BUS-P3-02
A Phase 3, 24-Week, Randomized, Double-Blind, Placebo-Controlled, Parallel-Arm Efficacy and Safety Study with Open-label Extension of BLU-5937 in Adult Participants with Refractory Chronic Cough Including Unexplained Chronic Cough (CALM-2)
-
Protocol number: C1071007-NCT05317416
A RANDOMIZED, 2-ARM, PHASE 3 STUDY OF ELRANATAMAB (PF-06863135) VERSUS LENALIDOMIDE IN PATIENTS WITH NEWLY DIAGNOSED MULTIPLE MYELOMA AFTER UNDERGOING AUTOLOGOUS STEM-CELL TRANSPLANTATION
-
Protocol number: C3300
A Phase 2 Randomized, Double-blind, Placebo-controlled, Adaptive Study to Evaluate the Efficacy, Safety, and Tolerability of 2 Dose Levels of IW-3300 Administered Rectally for 12 Weeks to Treat Bladder Pain in Subjects with Interstitial Cystitis/Bladder Pain Syndrome
-
Protocol number: C3651011
A PHASE 2, DOUBLE-BLIND, RANDOMIZED, PLACEBO-CONTROLLED, 4-ARM STUDY TO INVESTIGATE SYMPTOMS, FUNCTION, HEALTH-RELATED QUALITY OF LIFE AND SAFETY WITH REPEATED SUBCUTANEOUS ADMINISTRATION OF PONSEGROMAB VERSUS PLACEBO IN ADULT PARTICIPANTS WITH HEART FAILURE
-
Protocol number: C4391024-FOURLIGHT-3
AN INTERVENTIONAL, OPEN-LABEL, RANDOMIZED, MULTICENTER PHASE 3 STUDY OF PF-07220060 PLUS LETROZOLE COMPARED TO CDK4/6 INHIBITOR PLUS LETROZOLE IN PARTICIPANTS OVER 18 YEARS OF AGE WITH HORMONE RECEPTOR (HR)-POSITIVE, HER2-NEGATIVE ADVANCED/METASTATIC BREAST CANCER WHO HAVE NOT RECEIVED ANY PRIOR SYSTEMIC ANTICANCER TREATMENT FOR ADVANCED/METASTATIC DISEASE (FOURLIGHT-3)
-
Protocol number: C4551002-NCT07062965
AN INTERVENTIONAL, OPEN-LABEL, RANDOMIZED, MULTICENTER, PHASE 3 STUDY OF PF-07248144 PLUS FULVESTRANT COMPARED TO INVESTIGATOR CHOICE OF THERAPY IN ADULT PARTICIPANTS WITH HORMONE RECEPTOR-POSITIVE, HER2-NEGATIVE ADVANCED/METASTATIC BREAST CANCER WHOSE DISEASE PROGRESSED AFTER PRIOR CDK4/6 INHIBITOR-BASED THERAPY
-
Protocol number: C4951063
AN INTERVENTIONAL EFFICACY AND SAFETY, PHASE 3, DOUBLE-BLIND, PARALLEL-GROUP STUDY TO INVESTIGATE INTERMITTENT PREVENTION OF MENSTRUAL MIGRAINE WITH RIMEGEPANT COMPARED WITH PLACEBO IN WOMEN PARTICIPANTS 18 TO 45 YEARS OF AGE
-
Protocol number: C5091017
AN INTERVENTIONAL EFFICACY AND SAFETY, PHASE 3, DOUBLE-BLIND, 2-ARM STUDY TO INVESTIGATE ORALLY ADMINISTERED IBUZATRELVIR COMPARED WITH PLACEBO IN NON-HOSPITALIZED SYMPTOMATIC ADULT AND ADOLESCENT PARTICIPANTS WITH COVID-19 WHO ARE AT HIGH RISK OF PROGRESSING TO SEVERE ILLNESS
-
Protocol number: C5091018
AN INTERVENTIONAL EFFICACY AND SAFETY, PHASE 3, RANDOMIZED, DOUBLE-BLIND, 3-ARM STUDY TO INVESTIGATE IBUZATRELVIR IN ADULTS WITH SYMPTOMATIC COVID-19 WHO ARE SEVERELY IMMUNOCOMPROMISED
-
Protocol number: C5731006-NCT06966453
a new Phase 2 advanced breast cancer study and currently looking to identify qualified sites for this study to explore the efficacy and safety of Disitamab vedotin as a single agent in the HER2-positive, HER2-low, including HER2-low TNBC, and HER2 ultra-low patient populations.
-
Protocol number: CA073-1022-NCT06425302
A Phase 2 Randomized, Open Label Study to Evaluate the Efficacy and Safety of Golcadomide in Combination with Rituximab in Participants with Newly Diagnosed Advanced Stage Follicular Lymphoma
-
Protocol number: CAD-DECISIONAID
Shared Decision Making in the Prevention and Management of Cardiovascular Disease
-
Protocol number: CAPTIVA-NCT05047172
CAPTIVA is a two-stage Phase III trial randomizing subjects with stroke attributed to 70-99% intracranial atherosclerotic stenosis (sICAS) to: 1) ticagrelor + aspirin 2) low dose rivaroxaban + aspirin 3) clopidogrel + aspirin The primary goal of the trial is to determine if the experimental arm(s) (rivaroxaban or ticagrelor or both) are superior to the clopidogrel arm for lowering the 1-year rate of the primary endpoint (ischemic stroke, intracerebral hemorrhage (ICH), or vascular death). The first stage of the trial is concluded by an interim safety analysis intended to identify an excess of parenchymal intracerebral hemorrhage (ICH) or non-ICH major hemorrhage with ticagrelor + aspirin or low dose rivaroxaban + aspirin that could lead to an early termination of one or both of those arms. The second stage of the trial will determine if the experimental arm(s) (rivaroxaban or ticagrelor or both) that progress to stage 2 are superior to the clopidogrel arm for lowering the 1-year rate of the primary endpoint (ischemic stroke, intracerebral hemorrhage (ICH), or vascular death). An exploratory aim is to estimate the impact of CYP2C19 loss-of-function (LOF) carrier status on any benefit that the ticagrelor or low dose rivaroxaban arms may have in lowering the primary endpoint compared with the clopidogrel arm.
-
Protocol number: CARDIOL-100-102
Impact of CardiolRxTM on Myocardial Recovery in Acute Myocarditis
-
Protocol number: CARDUS-HANDHELD
Handheld Cardiac Ultrasound in Resident Assessment of Left Ventricular Ejection Fraction in Outpatients
-
Protocol number: CARILLON-NCT03142152
Assessment of the Carillon® Mitral Contour System® in Treating Functional Mitral Regurgitation Associated With Heart Failure - The CARILLON Trial
-
Protocol number: CASCADE-TESTING-STUDY00007418
Understanding Cascade Testing for Hereditary Cancer in Diverse Families
-
Protocol number: CATALYST-1073-310
Study of Hypercortisolism in Patients with Difficult to Control Type 2 Diabetes Despite Receiving Standard-of-Care Therapies: Prevalence and Treatment with Korlym® (Mifepristone) (CATALYST)
-
Protocol number: CATALYST-1073-310-GSM
Study of Hypercortisolism in Patients with Difficult to Control Type 2 Diabetes Despite Receiving Standard-of-Care Therapies: Prevalence and Treatment with Korlym® (Mifepristone) (CATALYST)
-
Protocol number: CATHBUDDY
PTC-006 CathBuddy, Inc. HLD Validation Through In-Use Testing Protocol
-
Protocol number: CB8025-41837-AFFIRM
AFFIRM: A Randomized, Double-Blind, Placebo-Controlled, Study to Evaluate the Effect of Seladelpar on Clinical Outcomes in Patients with Primary Biliary Cholangitis (PBC) and Compensated Cirrhosis
-
Protocol number: CB8025-41837-AFFIRM-GU
AFFIRM: A Randomized, Double-Blind, Placebo-Controlled, Study to Evaluate the Effect of Seladelpar on Clinical Outcomes in Patients with Primary Biliary Cholangitis (PBC) and Compensated Cirrhosis
-
Protocol number: CC-94676-PCA-001
A PHASE 1, MULTI-CENTER, OPEN-LABEL, DOSE-FINDING STUDY TO EVALUATE THE SAFETY, TOLERABILITY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF CC-94676 IN SUBJECTS WITH METASTATIC CASTRATION-RESISTANT PROSTATE CANCER
-
Protocol number: CD-O-101-NCT06699836
A Phase Two Study of Two Doses of Leronlimab (Pro 140) in Combination with Trifluridine + Tipiracil (TAS-102) + Bevacizumab in Participants with CCR5+, Microsatellite Stable (MSS), Relapsed Refractory Metastatic Colorectal Cancer (mCRC)
-
Protocol number: CHIMES-NCT04377555
AN OPEN-LABEL, MULTICENTER STUDY TO ASSESS DISEASE ACTIVITY AND BIOMARKERS OF NEURONAL DAMAGE IN MINORITY PATIENTS WITH RELAPSING MULTIPLE SCLEROSIS RECEIVING TREATMENT WITH OCRELIZUMAB
-
Protocol number: CLEE011O12001-MWHC-NCT05827081
A Phase IIIb study to characterize the effectiveness and safety of Adjuvant ribociclib in a wide patient population with HR+ HER2- early breast cancer (Adjuvant WIDER)
-
Protocol number: CLEE011O12005-REPOWER
"Real-world Evaluation of Patient Outcomes and Experiences with Ribociclib Early Adopters: A Hybrid Study with Prospective Patient-reported Outcomes and Retrospective Clinical Chart Review"
-
Protocol number: CLIN-10200-454
A multicentre, randomised, double-blind, placebo-controlled, dose escalation and dose finding phase II study to evaluate the safety and efficacy of IPN10200 in the prevention of episodic or chronic migraine in adults
-
Protocol number: CLIN-52120-463
A Phase III, Randomised, Double-blind, Placebo-controlled, Multicentre, Parallel-group Study with Extension Phase to Evaluate the Efficacy and Safety of Dysport® for the Prevention of Chronic Migraine in Adult Participants
-
Protocol number: CLIN-52120-464
A Phase III, Randomised, Double-blind, Placebo-controlled, Multicentre, Parallel-group Study with Extension Phase to Evaluate the Efficacy and Safety of Dysport® for the Prevention of Episodic Migraine in Adult Participants
-
Protocol number: CLNP023F12301
A multicenter, single-arm, open label trial to evaluate efficacy and safety of oral, twice daily LNP023 in adult aHUS patients who are naive to complement inhibitor therapy
-
Protocol number: CLOU064C12301
A randomized, double-blind, double-dummy, parallel-group study, comparing the efficacy and safety of remibrutinib versus teriflunomide in participants with relapsing multiple sclerosis, followed by extended treatment with open-label remibrutinib
-
Protocol number: CLR1806-NCT03655236
A PHASE 2, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDY OF K0706 IN SUBJECTS WITH EARLY PARKINSON S DISEASE
-
Protocol number: CM-TS01
A Phase III study to compare the efficacy and safety of TachoSil and Surgicel Original as an adjunct to control mild to moderate soft tissue bleeding during surgery
-
Protocol number: CO-US-979-6770-NCT06248515
A Phase II Trial of Sacituzumab Govitecan-Hziy in Patients with Advanced Thymic Epithelial Tumors
-
Protocol number: CO44657-NCT06065748
A PHASE III, RANDOMIZED, OPEN-LABEL STUDY EVALUATING EFFICACY AND SAFETY OF GIREDESTRANT COMPARED WITH FULVESTRANT, BOTH COMBINED WITH A CDK4/6 INHIBITOR, IN PATIENTS WITH ESTROGEN RECEPTOR-POSITIVE, HER2-NEGATIVE ADVANCED BREAST CANCER WITH RESISTANCE TO PRIOR ADJUVANT ENDOCRINE THERAPY
-
Protocol number: CO44668-MWHC-NCT05904886
A PHASE III, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDY EVALUATING ATEZOLIZUMAB AND BEVACIZUMAB, WITH OR WITHOUT TIRAGOLUMAB, IN PATIENTS WITH UNTREATED LOCALLY ADVANCED OR METASTATIC HEPATOCELLULAR CARCINOMA
-
Protocol number: COE-002-1116
Caris Molecular Intelligence® and Caris Centers of Excellence for Precision Medicine NetworkTM Retrospective Outcomes-Associated Database
-
Protocol number: COG0203
Synaptic Therapy Alzheimer's Research Trial (START): A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial to Evaluate the Safety and Efficacy of CT1812 in Early Alzheimer's Disease over 18 Months
-
Protocol number: COLOGUARD
A Real World Study of Patients Under the Age of 50 Screened for Colorectal Cancer (CRC) A Real World Study of Patients Under the Age of 50 Screened for Colorectal Cancer (CRC) Using Cologuard® in the U.S. Tidal
-
Protocol number: COMB157GUS10
AGNOS: An 18-month, Open-label, Multi-Center Study to Assess the Effect of Ofatumumab 20mg SC Monthly in Treatment Naïve, Very Early Relapsing Remitting Multiple Sclerosis Patients Benchmarked Against Healthy Controls on Select Outcomes.
-
Protocol number: COMB157Q12301
An open-label, randomized, parallel group, non-inferiority study to investigate the pharmacokinetics, pharmacodynamics, safety and tolerability of a new maintenance dosing regimen of ofatumumab, followed by extended treatment in participants with relapsing multiple sclerosis
-
Protocol number: COMIND
An Early Feasibility Study for the Development of a Model to Non-invasively Estimate Intracranial Pressure (ICP) and New Metrics of Cerebral Autoregulation (CAR) using the CoMind One EFS Device
-
Protocol number: CONFORM-NCT05147792
The CONFORM Pivotal Trial; An Evaluation of the Safety and Effectiveness of the Conformal CLAAS System for Left Atrial Appendage Occlusion
-
Protocol number: CONNECT-HF-NCT03035474
CARE OPTIMIZATION THROUGH PATIENT AND HOSPITAL ENGAGEMENT CLINICAL TRIAL FOR HEART FAILURE CONNECT-HF
-
Protocol number: CONTEGOPERFORMANCEIII
Protection against Emboli during caRotid artery stenting using a Neuroguard IEP® Direct 3-in-1 delivery system comprised oF a pOstdilation balloon, integRated eMbolic filter, and A Novel Carotid stEnt III
-
Protocol number: COOP
A Multi-Center, Prospective Non-Inferiority, Two-arm Randomized Trial Comparing the Effectiveness of Annual Fecal Immunochemical Testing vs. Colonoscopy in Adults Age 65-82 with History of Low-Risk Colorectal Polyps
-
Protocol number: CORCINCH-HF
Randomized Clinical Evaluation of the AccuCinch® Ventricular Repair System in Patients who Present with Symptomatic Heart Failure with Reduced Ejection Fraction (HFrEF)
-
Protocol number: COREVITASSPHERES-NCT04886492
CorEvitas SPHERES (Synergy of Prospective Health & Experimental Research for Emerging Solutions) Registry for Neuromyelitis Optica Spectrum Disorder (NMOSD)
-
Protocol number: COREVNS-NCT03529045
Comprehensive Outcomes Registry in Subjects with Epilspsy Treated with Vagus Nerve Stimulation Therapy
-
Protocol number: CORMATRIX-NCT02397668
CORMATRIX® COR TRICUSPID ECM® VALVE REPLACEMENT SAFETY AND EARLY FEASIBILITY STUDY
-
Protocol number: CORXEL-JX10002
Optimizing Reperfusion to Improve Outcomes and Neurologic Function (ORION): A Multicenter, Double-Blind, Placebo-Controlled, Randomized, Parallel-Group, Phase 2/3 Study to Evaluate the Efficacy and Safety of JX10 in Acute Ischemic Stroke with Late Presentations
-
Protocol number: CRETO-EAP
An Expanded Access Program of Cretostimogene Grenadenorepvec in Patients with Non-Muscle Invasive Bladder Cancer (NMIBC) Unresponsive to Bacillus Calmette-Guerin (BCG)
-
Protocol number: CRISPATUS
Intravesical Lactobacillus crispatus: Clinical Safety and Microbiome Evaluation
-
Protocol number: CSL889-2001
A Phase 2 / Phase 3, Multicenter, Randomized, Multiple-Dose, Double-Blind, Placebo-Controlled Adaptive Study to Evaluate the Safety, Efficacy, and Pharmacokinetics of CSL889 in Adults and Adolescents with Sickle Cell Disease during Vaso-Occlusive Crisis
-
Protocol number: CT7001-002
AN OPEN-LABEL INTERVENTIONAL, MULTICENTER, RANDOMIZED, PHASE 2 STUDY OF FULVESTRANT WITH OR WITHOUT SAMURACICLIB IN PARTICIPANTS WITH METASTATIC OR LOCALLY ADVANCED HORMONE RECEPTOR POSITIVE AND HUMAN EPIDERMAL GROWTH FACTOR RECEPTOR 2-NEGATIVE BREAST CANCER
-
Protocol number: CTHR24-001
Thrombophilia GEnetic STudy in the USA: Thrombophilia occurrence study in patients admitted to a thrombosis clinic.
-
Protocol number: CUTI
(Consumers) Assessment of SCI model systems complicated UTI consensus guidelines- Aim 4
-
Protocol number: CVRX-BAROSTIMTHERAPY
BAROSTIM THERAPY In Heart Failure with Reduced Ejection Fraction A Post-Market Registry with the BAROSTIM NEO System
-
Protocol number: CY6033-NCT06081894
AFICAMTEN (CK-3773274) PROTOCOL CY 6033 A PHASE 3, MULTI-CENTER, RANDOMIZED, DOUBLE-BLIND TRIAL TO EVALUATE THE EFFICACY AND SAFETY OF AFICAMTEN COMPARED TO PLACEBO IN ADULTS WITH SYMPTOMATIC NON-OBSTRUCTIVE HYPERTROPHIC CARDIOMYOPATHY
-
Protocol number: CYTOKINETICS-CY6022
An Open-Label Study of CK-3773274 for Patients with Symptomatic Hypertrophic Cardiomyopathy (HCM)
-
Protocol number: D3460C00002
A Multicenter, Randomized, Parallel-group, Double-blind, Two-arm, Phase III Study to Evaluate the Safety and Efficacy of Anifrolumab Compared with Placebo in Male and Female Participants 18 to 70 years of Age inclusive with Systemic Sclerosis
-
Protocol number: D4191C00140-NCT05771480
A Phase IIIb, Single Arm, Open-label, Multicentre Study of Durvalumab in Combination with Chemotherapy for the First Line Treatment for Patients with Advanced Biliary Tract Cancers
-
Protocol number: D419ML00003-TRITON-MWHC
A Phase IIIb, Randomized, Multicenter, Open-label Study to Assess the Efficacy of Durvalumab Plus Tremelimumab Versus Pembrolizumab in Combination With Platinum-Based Chemotherapy for First-Line Treatment in Metastatic Non-Small Cell Lung Cancer Patients With Non-Squamous Histology Who Have Mutations and/or Co-mutations in STK11, KEAP1, or KRAS (TRITON)
-
Protocol number: D7025C00001-NCT06109779
(BLINDED/UNBLINDED) A Phase III, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Global Study of Rilvegostomig in Combination With Chemotherapy as Adjuvant Treatment After Resection of Biliary Tract Cancer With Curative Intent
-
Protocol number: D798EC00001-EVOLVE
A Phase III, Randomized, Open-Label, Multi-Center, Global Study of Volrustomig (MEDI5752) as Sequential Therapy Versus Observation in Participants with Unresected Locally Advanced Head and Neck Squamous Cell Carcinoma, Who Have Not Progressed Following Definitive Concurrent Chemoradiotherapy (eVOLVE-HNSCC)
-
Protocol number: D8450R00003
A Non-interventional, Prospective, Multi-country Study Collecting Real-world Data on the Characteristics, Treatment Patterns, and Outcomes of Patients with Amyloid Transthyretin (ATTR) Amyloidosis
-
Protocol number: D926TC00001
A Phase III, Randomised, Open-label, Global Study of Adjuvant Datopotamab Deruxtecan (Dato-DXd) in Combination With Rilvegostomig or Rilvegostomig Monotherapy Versus Standard of Care, Following Complete Tumour Resection, in Participants With Stage I Adenocarcinoma Non-small Cell Lung Cancer who are ctDNA-positive or Have High-risk Pathological Features
-
Protocol number: D9802C00001-NCT06346392
A Phase III Multi-center, Open-label, Sponsor-blinded, Randomized Study of AZD0901 Monotherapy Compared with Investigator's Choice of Therapy in Second- or Later-Line Adult Participants with Advanced/Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma Expressing Claudin18.2
-
Protocol number: D9804C00001-NCT07069712
A Master Protocol for an Open-Label, Multi-Drug, Multi-Center, Phase II Platform Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Preliminary Anti-Tumor Activity of Novel Agents or Combinations as Perioperative Treatment in Participants with Locally Advanced Resectable Gastroesophageal Adenocarcinoma (GEMINI-PeriOp GC)
-
Protocol number: DAL-GENE-2
A phase III, double-blind, randomized placebo-controlled study to evaluate the effects of dalcetrapib on cardiovascular (CV) risk in a genetically defined population with a recent Acute Coronary Syndrome (ACS): The DAL-302 (dal-GenE-2) trial
-
Protocol number: DARANIVOVAX-2022-NCT06015724
A Phase 2 Study Evaluating the Efficacy of Anti-CD38 antibody in Combination with KRAS vaccine and Anti-PD-1 Antibody in Subjects with Pancreatic Ductal Adenocarcinoma and Refractory Non-Small Cell Lung Cancer
-
Protocol number: DB-1303-O-1001-NCT05150691
A Phase 1/2a Multicenter, Open-Label, Non-Randomized First in Human Study to Assess the Safety, Tolerability, Pharmacokinetics, and Preliminary Antitumor Activity of DB-1303 in Patients with Advanced/Metastatic Solid Tumors
-
Protocol number: DB-1419-O-1001-NCT06554795
A Phase 1/2a Study of DB-1419 in Advanced/Metastatic Solid Tumors - Temp
-
Protocol number: DB-EF-PHASEIII-0001
Bone Marrow Mesenchymal Stem Cell Derived Extracellular Vesicles for Hospitalized Patients with Respiratory Failure due to Severe or Critical COVID-19, Including Those with Moderate-to-Severe ARDS: A Phase III Clinical Trial.
-
Protocol number: DCM-CMR-STUDY
Dilated Cardiomyopathy (DCM) Research Project Sub-study: Advanced Procedures Sub-Study
-
Protocol number: DDD-SPECIMEN
Extracellular vesicles produced by CRISPR-activated MSCs: A potential therapy for degenerative disc disease
-
Protocol number: DENOVO
De Novo Autoimmune Hepatitis in Pediatric Liver Transplantation
-
Protocol number: DEXCOM-PTL-1000173
Evaluation of the Dexcom Continuous Glucose Monitoring (CGM) System in Pregnancy.
-
Protocol number: DEXTERITY-CIP0217
DEXTERITY-AFP: Perivenous Dexamethasone Therapy: Examining Reduction of Inflammation after Thrombus Removal to Yield Benefit in Acute Femoropopliteal DVT
-
Protocol number: DISCOVER-HCM
Observational Study Protocol CV027-012: DELIVER INSIGHTS IN HYPERTROPHIC CARDIOMYOPATHY AND OBSERVATIONAL OUTCOMES IN REAL WORLD (DISCOVER-HCM): UNITED STATES PROSPECTIVE REGISTRY STUDY
-
Protocol number: DISRUPT-CAD-DUO
Prospective, Multicenter, Single-Arm IDE Study of the Shockwave Coronary Intravascular Lithotripsy (IVL) System with the Shockwave C2+ 2Hz Coronary IVL Catheter in Calcified Coronary Arteries (Disrupt CAD Duo Study)
-
Protocol number: DNTH103-CIDP-301
A PHASE 3 RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDY TO EVALUATE THE EFFICACY AND SAFETY OF DNTH103 IN ADULTS WITH CHRONIC INFLAMMATORY DEMYELINATING POLYNEUROPATHY (CAPTIVATE)
-
Protocol number: DOD-EARLY-CAREER
Patient and Caregiver Perspectives on Intravesical Instillations for Urinary Symptoms
-
Protocol number: DOTS
Food and Drug Administration (FDA) Toxicology Investigator's Consortium (ToxIC) Drug Overdose Toxico-Surveillance (DOTS) Reporting Program.
-
Protocol number: DRESSINGCOMPARRISON
A Comparison of Split-thickness Skin Graft Primary Dressings When Used with Autologous Skin Cell Suspension.
-
Protocol number: DUET-CD
A Phase 2b Randomized, Double-blind, Active- and Placebo-controlled, Parallel-group, Multicenter Study to Evaluate the Efficacy and Safety of Induction and Maintenance Combination Therapy with Guselkumab and Golimumab in Participants with Moderately to Severely Active Crohn’s Disease”
-
Protocol number: DUET-UC
A Phase 2b Randomized, Double-blind, Active- and Placebo-controlled, Parallel-group, Multicenter Study to Evaluate the Efficacy and Safety of Induction and Maintenance Combination Therapy with Guselkumab and Golimumab in Participants with Moderately to Severely Active Ulcerative Colitis.
-
Protocol number: E01-RESUSCITATION
Pilot Multicenter Study of Resuscitation with Enteral Fluids
-
Protocol number: EA2185-PANCREATIC-NCT04239573
Comparing Two Methods to Follow Patients With Pancreatic Cysts
-
Protocol number: EA5231-NCT06732401
A Randomized Phase III Trial of Checkpoint Blockade in Lung Cancer Patients in the Adjuvant Setting Based on Pathologic Response Following Neoadjuvant Therapy (CLEAR)
-
Protocol number: EASESBS2EXT-NCT03905707
A Double-Blind Phase 3 Extension Trial Assessing the Long-Term Safety and Efficacy of Glepaglutide in Patients with Short Bowel
-
Protocol number: EASESBS3EXT-NCT04991311
A 104-week, multicenter, single-arm, long-term, phase 3 extension trial investigating the safety and efficacy of Glepaglutide in adult patients with short bowel syndrome (SBS) completing the EASE SBS 2 trial
-
Protocol number: EASI-KIDNEY
A multicenter international randomized double-blind placebo-controlled clinical trial of the aldosterone synthase inhibitor BI 690517 in patients with chronic kidney disease treated with empagliflozin
-
Protocol number: EAST-002
Randomized Controlled Clinical Trial (RCT) of the Nectero EAST System for Small to Mid-Sized Abdominal Aortic Aneurysms (AAA) StaBiLization: Evaluation of Efficacy.
-
Protocol number: EBONI
ViiV Healthcare / “A phase 4, randomized, open-label, three arm study to evaluate implementation strategies for the delivery of CAB for HIV Pre-exposure Prophylaxis (PrEP) across clinical settings for adult (≥18 years) Black cis-and transgender women without HIV infection living in the United States Ending the Epidemic (EHE) territories”
-
Protocol number: EIDOSACT-EARLY
A Phase 3, Randomized, Multicenter, Double-Blind, Placebo-Controlled Study of Acoramidis for Transthyretin Amyloidosis Prevention in the Young (ACT-EARLY Trial)
-
Protocol number: EIK1001-005-FSQ
A Phase 2 Study of EIK1001 in Combination with Pembrolizumab and Chemotherapy in Patients with Stage 4 Non-Small Cell Lung Cancer
-
Protocol number: EIP21-NFD-504
A Phase 2b Clinical Study of the P38 Alpha Kinase Inhibitor Neflamapimod in Patients with Dementia with Lewy Bodies (DLB)
-
Protocol number: EKOBLANKETPROTOCOL
Evaluation of Eko hardware, software, and algorithm performance across multiple scenarios, use cases, and device generations
-
Protocol number: ELA026-CP002-2/3
A Multipart, Open-label, Single-arm, Multicenter Study to Evaluate the Safety, Efficacy, and Pharmacokinetics of ELA026 in Participants with Secondary Hemophagocytic Lymphohistiocytosis (sHLH)
-
Protocol number: ELEGANCE-REGISTRY
ELEGANCE Drug-ELuting REGistry: ReAl-World Treatment of LesioNs in the Peripheral VasCulaturE
-
Protocol number: EMBLOK
A Prospective, Randomized, Multicenter Evaluation of the Safety and Effectiveness of the EMBLOK™ Embolic Protection System during Transcatheter Aortic Valve Replacement
-
Protocol number: ENLIGHTEN-NCT04140305
A Multicenter, Longitudinal, Open-Label, Single-Arm Study Describing Cognitive Processing Speed Changes in Relapsing Multiple Sclerosis Subjects Treated With Ozanimod (RPC-1063)
-
Protocol number: ENROLL-HD
Enroll-HD: A Prospective Registry Study in a Global Huntington s Disease Cohort A CHDI Foundation Project
-
Protocol number: ENVISION
Evaluation of the Navitor Transcatheter Heart Valve in Low and Intermediate Risk Patients who have Severe, Symptomatic, Aortic Stenosis Requiring Aortic Valve Replacement
-
Protocol number: EP0031-101
A Modular, Open-label, Phase I/II Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of EP0031 in Patients With Advanced RET-altered Malignancies
-
Protocol number: EPITOME-CLIN-52120-458-NCT0605
A PROSPECTIVE, MULTICOUNTRY STUDY TO ESTIMATE THE INCIDENCE OF AND PROVIDE A BEST PRACTICE MODEL FOR MONITORING THE DEVELOPMENT OF POST-STROKE SPASTICITY
-
Protocol number: EQU-202
A Randomized Phase 2 Study of Adjunctive EQU-001 for Uncontrolled Focal Onset Seizures
-
Protocol number: ESCAPE-MEVO
A Multicentre, Prospective, Randomized, Parallel Group, Open-label Design to Determine the Efficacy and Safety of Endovascular Thrombectomy for ischemic stroke patients with symptomatic Acute Medium Vessel Intracranial Occlusions
-
Protocol number: ESR-18-13870
A Phase II Trial of Durvalumab (MEDI4736)and Tremelimumab in Combination With Chemotherapy in HIV-infected Patients With Non-small Cell Lung Cancer
-
Protocol number: ETCTN-10204-NCT03816345
A Phase Ib Study of Nivolumab in Patients with Autoimmune Disorders and Advanced Malignancies (AIM-NIVO).
-
Protocol number: ETCTN-10276-NCT04068194
A Phase I/II Study of M3814 and Avelumab in Combination with Hypofractionated Radiation in Patients with Advanced/Metastatic Solid Tumors and Hepatobiliary Malignancies
-
Protocol number: ETCTN-10504-NCT05333458
A Phase 2 Study of Atezolizumab with or Without Selinexor in Alveolar Soft Part Sarcoma (AXIOM)
-
Protocol number: ETCTN-10629-NCT06287775
A Phase I Dose Finding and Phase II Randomized Trial of Iadademstat Combined With Immune Checkpoint Inhibition Maintenance After Initial Chemoimmunotherapy in Patients With Extensive-Stage Small Cell Lung Cancer
-
Protocol number: ETCTN-10699-NCT06860594
A Phase I Trial Combining Triapine With Radiation Therapy for Recurrent Glioblastoma or Astrocytoma
-
Protocol number: EV-ICD-CAS
ExtraVascular Implantable Cardiac Defibrillator (EV ICD) Continued Access Study
-
Protocol number: EXA2202-NCT05796999
ExaStim Upper Limb Pivotal Clinical Validation Study
-
Protocol number: EXPAND-NCR235148
Expanding and Promoting Alternative Care and Knowledge in Decision-Making: The ExPAND Study (Improving Shared Decision- Making and Access to Non-Dialytic Treatment for People with Kidney Disease)
-
Protocol number: EXTEND-001
Thoraflex Hybrid and Relay Extension Post-Approval Study (EXTEND)
-
Protocol number: F60089IV101-NCT06669117
A First-In-Human (FIH) phase I/II open-label, multicentre, dose escalation and expansion trial of VERT-002 in patients with locally advanced or metastatic solid tumors including non-small cell lung cancer (NSCLC) harboring MET alterations.
-
Protocol number: FASTR-RCV-0006
Fluid management of Acute decompensated heart failure Subjects Treated with Reprieve Decongestion Management System (DMS)
-
Protocol number: FENTREPID
A PHASE III MULTICENTER, RANDOMIZED, DOUBLE-BLIND, DOUBLE-DUMMY, PARALLEL-GROUP STUDY TO EVALUATE THE EFFICACY AND SAFETY OF FENEBRUTINIB COMPARED WITH OCRELIZUMAB IN ADULT PATIENTS WITH PRIMARY PROGRESSIVE MULTIPLE SCLEROSIS
-
Protocol number: FORWARD-IDE
Forward-Shifted Intravascular Lithotripsy (IVL) Technology in a Prospective, Multi-center, Single-arm Investigational Device Exemption (IDE) Study (FORWARD IDE Study)
-
Protocol number: FORWARDCAD
Prospective, Multicenter, Single-Arm, Investigational Device Exemption (IDE) Study of the Shockwave Intravascular Lithotripsy (IVL) System with the Shockwave Javelin Coronary IVL Catheter
-
Protocol number: G-LEAGUE
Understanding the Impact of a G League Season on Player Health, Performance and Patellar Tendinopathy: A Pilot Observational Study
-
Protocol number: GA43360
A Phase 1b, Double-Blind, Randomized, Placebo-Controlled, Multicenter Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Multiple-Ascending Doses of RO7303509 in Patients with Systemic Sclerosis
-
Protocol number: GAE-P3-22-01
Multicenter, PrOspective, Randomized, Controlled Trial Comparing GenIcular Artery EmbOlization Using Embosphere Microspheres to Corticosteroid iNjections for the Treatment of Symptomatic Knee Osteoarthritis: MOTION Study
-
Protocol number: GAE-P3-22-01-NCT05818150
Multicenter, PrOspective, Randomized, Controlled Trial Comparing GenIcular Artery EmbOlization Using Embosphere Microspheres to Corticosteroid iNjections for the Treatment of Symptomatic Knee Osteoarthritis: MOTION Study
-
Protocol number: GENENTECHML43702
LONG-TERM FOLLOW-UP STUDY OF PATIENTS WITH SPINAL MUSCULAR ATROPHY RECEIVING RISDIPLAM TREATMENT
-
Protocol number: GENERATION1-NCT02565511
A Randomized, Double-blind, Placebo-controlled, Two-cohort, Parallel Group Study to Evaluate the Efficacy of CAD106 and CNP520 in Participants at Risk for the Onset of Clinical Symptoms of Alzheimer's Disease.
-
Protocol number: GMS-PH-401
A Real-world Patient Registry in Group 3 Pulmonary Hypertension Associated With Interstitial Lung Disease (PH-ILD)
-
Protocol number: GN42272
A PHASE III MULTICENTER, RANDOMIZED, DOUBLE-BLIND, DOUBLE-DUMMY, PARALLEL-GROUP STUDY TO EVALUATE THE EFFICACY AND SAFETY OF FENEBRUTINIB COMPARED WITH TERIFLUNOMIDE IN ADULT PATIENTS WITH RELAPSING MULTIPLE SCLEROSIS
-
Protocol number: GO42216
A PHASE Ib/II, OPEN-LABEL, MULTICENTER, RANDOMIZED UMBRELLA STUDY EVALUATING THE EFFICACY AND SAFETY OF MULTIPLE IMMUNOTHERAPY-BASED TREATMENT COMBINATIONS IN PATIENTS WITH ADVANCED LIVER CANCERS (MORPHEUS-LIVER)
-
Protocol number: GO43878-NCT06084936
A Phase III, Open-Label, Multicenter Randomized Study Evaluating Glofitamab as a Single Agent Versus Investigator's Choice in Patients With Relapsed/Refractory Mantle Cell Lymphoma
-
Protocol number: GO44457-NCT04524871
A PHASE Ib/II, OPEN-LABEL, MULTICENTER, RANDOMIZED PLATFORM STUDY EVALUATING THE EFFICACY AND SAFETY OF NEOADJUVANT IMMUNOTHERAPY COMBINATIONS IN PATIENTS WITH SURGICALLY RESECTABLE HEPATOCEULLULAR CARCINOMA
-
Protocol number: GORE-VIAFORT-NCT05409976
Evaluation of the GORE® VIAFORT Vascular Stent for Treatment of Symptomatic Inferior Vena Cava Obstruction with or without Combined Iliofemoral Obstruction
-
Protocol number: GORETAMBEPAS
GORE® EXCLUDER® Thoracoabdominal Branch Endoprosthesis Post-Approval Study
-
Protocol number: GP-88
Serum GP-88 Levels in Metastatic Breast Cancer patients: A Prospective Blood Sampling Trial
-
Protocol number: GS-US-425-6143
GS-US-425-6143 - A Phase 1b Randomized, Blinded, Placebo-Controlled, Single and Multiple Ascending Dose Study to Evaluate the Safety, Tolerability and Pharmacokinetics of GS-8588 in People with HIV-1 who are Virologically Suppressed on Antiretroviral Therapy
-
Protocol number: GS-US-563-5925
A Phase 3, Randomized, Double-blind, Active-controlled Study to Evaluate a Switch to an Oral Weekly Islatravir/Lenacapavir Regimen in People With HIV-1 Who Are Virologically Suppressed on Bictegravir/Emtricitabine/Tenofovir (B/F/TAF)
-
Protocol number: GS-US-563-5926
A Phase 3, Randomized, Open-Label, Active-Controlled Study to Evaluate a Switch to an Oral Weekly Islatravir/Lenacapavir Regimen in People With HIV-1 Who Are Virologically Suppressed on Standard of Care
-
Protocol number: GS-US-595-6184-ASCENT-05
A Randomized, Open-label, Phase 3 Study of Adjuvant Sacituzumab Govitecan and Pembrolizumab Versus Treatment of Physician s Choice in Patients With Triple Negative Breast Cancer Who Have Residual Invasive Disease After Surgery and Neoadjuvant Therapy
-
Protocol number: GS-US-595-6184-NCT05633654
A Randomized, Open-label, Phase 3 Study of Adjuvant Sacituzumab Govitecan and Pembrolizumab Versus Treatment of Physician's Choice in Patients With Triple Negative Breast Cancer Who Have Residual Invasive Disease After Surgery and Neoadjuvant Therapy
-
Protocol number: GS-US-685-6819
A Phase 2 Randomized, Placebo-controlled Study of the Safety and Efficacy of Obeldesivir to Treat Nonhospitalized Adults With Acute Respiratory Syncytial Virus (RSV) Infection
-
Protocol number: GS-US-695-6509
An Operationally Seamless Phase 2/3, Randomized, Active-Controlled Study Evaluating the Safety and Efficacy of an Oral Weekly Regimen of GS-1720 in Combination With GS-4182 Versus Biktarvy in Virologically Suppressed People With HIV-1
-
Protocol number: GS-US-695-7156
An Operationally Seamless Phase 2/3, Randomized, Active-Controlled Study Evaluating the Safety and Efficacy of an Oral Weekly Regimen of GS-1720 in Combination With GS-4182 Versus Biktarvy in Treatment-Naive People With HIV-1
-
Protocol number: GSK-219369-AZUR-1
A Phase 2, Single-Arm, Open-Label Study with Dostarlimab Monotherapy in Participants with Untreated Stage II/III dMMR/MSI-H Locally Advanced Rectal Cancer
-
Protocol number: GSK-219606-AZUR-2
A Phase 3, Open-Label, Randomized Study of Perioperative Dostarlimab Monotherapy versus Standard of Care in Participants with Untreated T4N0 or Stage III dMMR/MSI-H Resectable Colon Cancer
-
Protocol number: GUH-CDF-STUDY
Feasibility Study to Understand Screening and Treatment Rates for Cognitive Impairment, Depression, and Fatigue in a Large, Urban MS Comprehensive Care Center
-
Protocol number: HBVFB
Impact of Recent Immigration on Delays in Care Delivery among Foreign-Born with Chronic Hepatitis B Infection
-
Protocol number: HCRN-GU22-587-NCT05928806
Advanced Renal Cell Cancer Combination ImmunoThErapy Clinical Trial (ARCITECT)
-
Protocol number: HEAL-IST
Hybrid Epicardial and Endocardial Sinus-Node SpAring AbLation Therapy for Inappropriate Sinus Tachycardia
-
Protocol number: HEM-PWRV1US
HEM-POWR: Observational Study Evaluating Effectiveness and Safety of Real-World Treatment with Damoctocog alfa pegol in Previously Treated Patients with Hemophilia A
-
Protocol number: HEP3
Human Epilepsy Project 3: Newly Diagnosed Idiopathic Generalized Epilepsy
-
Protocol number: HIALIVER-NCT03734393
HOPE in Action Prospective Multicenter, Clinical Trial of HIV+ Deceased Donor Liver Transplants for HIV+ Recipients
-
Protocol number: HIBISCUS-2
A global phase 3, randomised, double-blind and placebo-controlled study evaluating the efficacy and safety of etavopivat in adolescents and adults with sickle cell disease
-
Protocol number: HIT/AIH
Enhancing Functional Recovery: Acute Intermittent Hypoxia and High-Intensity Gait Training
-
Protocol number: HP-00081403-NCT03652428
Phase I Study of Concurrent Nab-Paclitaxel + Gemcitabine With Hypofractionated, Ablative Proton Therapy for Locally Advanced Pancreatic Cancer
-
Protocol number: HSG001
Development of the Virtual Unified Huntington Disease Rating Scale (vUHDRS)
-
Protocol number: I-SPY2-NCT01042379
I-SPY Trial (Investigation of Serial Studies to Predict Your Therapeutic Response With Imaging And moLecular Analysis 2)
-
Protocol number: I5T-MC-AACI
Assessment of Safety, Tolerability, and Efficacy of Donanemab in Early Symptomatic Alzheimer's Disease
-
Protocol number: IBD-QORUS-GU
IBD Qorus: Improving the Quality of Care for Adults with Inflammatory Bowel Disease
-
Protocol number: ICG-SCAPHOID
Use of Indocyanine Green intraoperatively to examine vascularity of scaphoid with histological comparison
-
Protocol number: IGC-AD1-P2BIDAG
A Phase 2, Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Trial of the Safety and Efficacy of IGC-AD1 on Agitation in Participants with Dementia due to Alzheimer's Disease.
-
Protocol number: IIRR-01164-ARTHREX
Outcomes of Nanoscopic Partial Meniscectomy Versus Standard Arthroscopic Partial Meniscectomy
-
Protocol number: IM027-068
A Multicenter, Randomized, Double-blind, Placebo-controlled, Phase 3 Study to Evaluate the Efficacy, Safety, and Tolerability of BMS-986278 in Participants with Idiopathic Pulmonary Fibrosis
-
Protocol number: IM027-1015
A Multicenter, Randomized, Double-blind, Placebo-controlled, Phase 3 Study to Evaluate the Efficacy, Safety, and Tolerability of BMS-986278 in Participants with Progressive Pulmonary Fibrosis
-
Protocol number: IMC-F106C-301-NCT06112314
A Phase 3 Randomized, Controlled Study of IMC-F106C Plus Nivolumab Versus Nivolumab Regimens in HLA-A*02:01-Positive Participants With Previously Untreated Advanced Melanoma
-
Protocol number: IMMUKNOW
Utility of ImmuKnow, an Immune Cell Function Assay, in a Cardiac Sarcoid Population
-
Protocol number: IMPACT2-PARK
IN-HOME STUDY WITH MINIMED; 780G PUMP AUTOMATED CONTROL IN TYPE 2 - EVALUATION OF THE AHCL SYSTEM IN ADULTS WITH INSULIN-REQUIRING TYPE 2 DIABETES.
-
Protocol number: IMPROVE-NCT04221815
IMPact on Revascularization Outcomes of intraVascular ultrasound guided treatment of complex lesions and Economic impact (IMPROVE)
-
Protocol number: IMPROVE-REL025
Intracept Minimally-invasive PROcedure for VErtebrogenic back pain (IMPROVE)
-
Protocol number: IMPROVEMENT-NCT705289609
International, Multicenter, Prospective, Non-competitive, Observational study to Validate and Optimize prediction models of 90-day and 1-year allograft failure after liver transplantation
-
Protocol number: IMVT-1401-2401
A Phase 2b, Multi-center, Randomized, Quadruple-blind, Placebo-controlled Study of Batoclimab Treatment in Adult Participants with Active Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
-
Protocol number: IMVT-1402-2502
A Randomized, Double-Blind, Placebo-Controlled, Phase 2b Study to Assess the Efficacy, Safety, and Tolerability of IMVT-1402 as Treatment for Adult Patients With Graves' Disease.
-
Protocol number: INS-416
ENCORE - A Randomized, Double-Blind, Placebo-Controlled, Active Comparator, Multicenter Study to Evaluate the Efficacy and Safety of an Amikacin Liposome Inhalation Suspension (ALIS)-Based Regimen in Adult Subjects with Newly Diagnosed Nontuberculous Mycobacterial (NTM) Lung Infection Caused by Mycobacterium avium Complex (MAC)
-
Protocol number: INSIGHT018-A
A Multicenter, Adaptive, Randomized, Controlled Trial Platform To Evaluate Safety and Efficacy of Strategies and Treatments for Hospitalized Patients with Respiratory Infections
-
Protocol number: INSIGHT018-B
University of Minnesota/ INSIGHT / “A Multicenter, Adaptive, Randomized, Controlled Trial Platform To Evaluate Safety and Efficacy of Strategies and Treatments for Hospitalized Patients with Respiratory Infections”
-
Protocol number: INSMED312-NCT02628600
An Open-Label Safety Extension Study to a Multicenter Study of Liposomal Amikacin for Inhalation (LAI) in Adult Patients with Nontuberculous Mycobacterial (NTM) Lung Infections caused by Mycobacterium avium complex (MAC) that are refractory to treatment
-
Protocol number: INTRAVESICALLGGVERSUSSALINE
Intravesical Lactobacillus rhamnosusGG versus saline bladder wash: A randomized, controlled, comparative effectiveness clinical trial
-
Protocol number: ION-682884-CS12
An Open-Label Extension Study to Assess the Long-Term Safety of Eplontersen (ION-682884) in Patients with Transthyretin-Mediated Amyloid Cardiomyopathy (ATTR-CM)
-
Protocol number: IPX203-401-23
Open-Label Phase 4 Study of CREXONT® (Carbidopa and Levodopa) Extended-Release Capsules in Parkinson’s Disease Patients
-
Protocol number: ISPYCOVID
I-SPY COVID TRIAL: An Adaptive Platform Trial to Reduce Mortality and Ventilator Requirements for Critically Ill Patients
-
Protocol number: ISS-59074-PEMBRO-LENV
A phase II trial of combination therapy of pembrolizumab and Lenvatinib in patients with locally advanced or metastatic cervical cancer
-
Protocol number: ISTRADEFYLLINECHARTREVIEW
Effectiveness of Istradefylline on Motor Functions in Patients with Parkinson's Disease
-
Protocol number: J2J-MC-JZLH-EMBER-4
EMBER-4: A Randomized, Open-Label, Phase 3 Study of Adjuvant Imlunestrant vs Standard Adjuvant Endocrine Therapy in Patients who have Previously Received 2 to 5 years of Adjuvant Endocrine Therapy for ER+, HER2- Early Breast Cancer with an Increased Risk of Recurrence
-
Protocol number: J2N-MC-JZNZ
A Phase 1/2, Dose-finding Study Investigating the Safety and Efficacy of Pirtobrutinib in Adults with Immune Thrombocytopenia
-
Protocol number: J3L-MC-EZEF
A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Effect of Lepodisiran on the Reduction of Major Adverse Cardiovascular Events in Adults with Elevated Lipoprotein(a) who have Established Atherosclerotic Cardiovascular Disease or Are at Risk for a First Cardiovascular Event ACCLAIM-Lp
-
Protocol number: J3L-MC-EZEF-GHAZI
A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Effect of Lepodisiran on the Reduction of Major Adverse Cardiovascular Events in Adults with Elevated Lipoprotein(a) who have Established Atherosclerotic Cardiovascular Disease or Are at Risk for a First Cardiovascular Event ACCLAIM-Lp
-
Protocol number: J3R-MC-YDAG
A Phase 3 Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Efficacy and Safety of Once Weekly Eloralintide in Adult Participants with Obesity or Overweight, without Type 2 Diabetes (ENLIGHTEN-1)
-
Protocol number: J3R-MC-YDAG-MGSH
A Phase 3 Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Efficacy and Safety of Once Weekly Eloralintide in Adult Participants with Obesity or Overweight, without Type 2 Diabetes (ENLIGHTEN-1)
-
Protocol number: JAGUAR-CP-0017
ObJective Analysis to GaUge EVAR Outcomes Through Randomization
-
Protocol number: JANSSEN-70033093STR3001
A Phase 3, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study to Demonstrate the Efficacy and Safety of Milvexian, an Oral Factor XIa Inhibitor, for Stroke Prevention after an Acute Ischemic Stroke or High-Risk Transient Ischemic Attack
-
Protocol number: JENAVALVE-AR-NCT02732704
JenaValve Pericardial TAVR Aortic Regurgitation Study
-
Protocol number: JENAVALVE-AS-NCT02732691
JenaValve Pericardial TAVR System Aortic Stenosis Study
-
Protocol number: JIDDU
Validating the Jiddu Analyzer for Urine Bacterial Growth in Neurogenic Lower Urinary Tract Dysfunction
-
Protocol number: JNJ-70033093
A Phase 3, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study to Demonstrate the Efficacy and Safety of Milvexian, an Oral Factor XIa Inhibitor, for Stroke Prevention after an Acute Ischemic Stroke or High-Risk Transient Ischemic Attack
-
Protocol number: JUGGERKNOT-PMS
JuggerKnot with BroadBand Tape Post Market Clinical Follow-up (PMCF) Study
-
Protocol number: JUVEENA
Safety and Effectiveness of Juveena Hydrogel System Following Transcervical Gynecologic Procedures (TCGP) At High-Risk for Intrauterine Adhesions: A Multicenter Randomized Controlled Subject and Evaluator Blinded Pivotal Study.
-
Protocol number: JZP598-303-MWHC-NCT06435429
Phase 3, randomized, open-label, multicenter, controlled study to evaluate the efficacy and safety of zanidatamab in combination with physicians choice chemotherapy compared to trastuzumab in combination with physicians choice chemotherapy for the treatment of participants with metastatic HER2-positive breast cancer who have progressed on, or are intolerant to, previous trastuzumab deruxtecan treatment
-
Protocol number: JZP598-303-NCT06435429
Phase 3, randomized, open-label, multicenter, controlled study to evaluate the efficacy and safety of zanidatamab in combination with physician's choice chemotherapy compared to trastuzumab in combination with physician's choice chemotherapy for the treatment of participants with metastatic HER2-positive breast cancer who have progressed on, or are intolerant to, previous trastuzumab deruxtecan treatment
-
Protocol number: KIDNEY-OCT-NCT00000000
Automatic Wide-Field Optical Coherence Tomography for Assessment of Transplant Kidney Viability
-
Protocol number: KRT-232-115
A Phase 3, Randomized, Double-blind, Add-on Study Evaluating the Safety and Efficacy of Navtemadlin Plus Ruxolitinib vs Placebo Plus Ruxolitinib in JAK Inhibitor-Naïve Patients with Myelofibrosis Who Have a Suboptimal Response to Ruxolitinib
-
Protocol number: KT-US-982-5968-NCT05041309
Long-term Follow-up Study for Participants of Kite-Sponsored Interventional Studies Treated With Gene-Modified Cells
-
Protocol number: KUPFER-BM-3680
Collection of blood and bone marrow from patients with genetic blood and bone marrow disease
-
Protocol number: LACTATESENSOR
SENSA-HF: A Sensor-Based Monitoring System for the Measurement of Lactate in Acute Decompensated Heart Failure – A Feasibility Study
-
Protocol number: LADD
Evaluation of Laser-assisted Drug Delivery for the Treatment of Hypertrophic Scar Hypopigmentation: A Within Patient-Controlled Trial in Skin of Color
-
Protocol number: LEAAPS-CP-2021-05
Left Atrial Appendage Exclusion for Prophylactic Stroke Reduction Trial
-
Protocol number: LEARN-STUDY00006767
Functional anomaly mapping of post-stroke aphasia recovery
-
Protocol number: LIMB-ISCHEMIA
Prospective Study Evaluating the Effect of Skin Pigmentation on Clinical Exam for Determination of Limb Ischemia in Patients with Digital Injuries
-
Protocol number: LITERACY
Limited Health Literacy in Patients with Rotator Cuff Tears: Risk Factors and Outcome Effects
-
Protocol number: LPX-641-001
A multicenter observational study for Immune-phenotyping and ex-vivo evaluation of the likelihood of response to an experimental tolerance restoration therapy in subjects diagnosed with multiple sclerosis (MS).
-
Protocol number: LUMINA-A114-001
A Phase 1, Randomized, Double-blind, Placebo-controlled, Multiple Ascending Dose Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Antisense Oligonucleotide AMX0114 Administered to Adult Participants with Amyotrophic Lateral Sclerosis
-
Protocol number: LUNDBECK18252A
Interventional, open-label, exploratory study, investigating the safety, tolerability, pharmacokinetics, and efficacy of Lu AF28996 in patients with Parkinson's disease
-
Protocol number: M11-001
An Observational, Non- Interventional, Multi-Center, Multi-National Study of Patients with Atypical Hemolytic-Uremic Syndrome
-
Protocol number: M19-148
A Randomized, Double-Blind, Placebo-Controlled Proof-of-Concept Study to Assess the Safety and Efficacy of Elezanumab in Acute Ischemic Stroke
-
Protocol number: M20-098
Advanced Parkinson's Disease: An Open-Label Extension of Studies M15-736 and M20-339 Evaluating the Safety and Tolerability of ABBV-951
-
Protocol number: M21-307/NCT05028569
Episodic Migraine: Phase 3 Study of BOTOX (Botulinum Toxin Type A) for the Prevention of Migraine in Subjects with Episodic Migraine
-
Protocol number: MAGIC-NCT05361941
Multicenter Trial of Antibiotic Eluting Graft for Promoting New Bone Growth In/​near Infected Bone Cavities (MAGIC)
-
Protocol number: MAPLE-NCT04793360
Molecular Assessment and Profiling of Liver Transplant Recipients
-
Protocol number: MATERNA-PREP
EASE Study: A randomized, controlled study to EvAluate the Safety and Effectiveness of the Materna Prep Device to pre-stretch the vaginal canal and pelvic floor muscles in preparation for vaginal delivery
-
Protocol number: MCLA-158-CL01-NCT03526835
Phase 1/2 Dose Escalation and Cohort Expansion Study Evaluating MCLA-158 (Petosemtamab) as Single Agent or in Combination in Advanced Solid Tumors
-
Protocol number: MCLA-158-CL02
A phase 3 open-label, randomized, controlled study to evaluate the efficacy and safety of petosemtamab compared with investigator s choice monotherapy treatment in previously treated patients with incurable, metastatic/recurrent head and neck squamous cell carcinoma
-
Protocol number: MCLA-158-CL03
A phase 3 randomized, open-label study to evaluate the efficacy and safety of petosemtamab plus pembrolizumab vs pembrolizumab in first-line treatment of recurrent or metastatic PD-L1+ head and neck squamous cell carcinoma.
-
Protocol number: MICROABPILOT
Characterizing the Postmenopausal Genitourinary Microbiome in Women undergoing Onabotulinum Toxin Type A forOveractive Bladder ; A Feasibility Trial
-
Protocol number: MITRAL2-NCT04408430
The safety and effectiveness of the SAPIEN 3 and SAPIEN 3 Ultra valve with Commander delivery system in patients with symptomatic severe calcific mitral valve disease with severe mitral annular calcification who are not candidates for standard mitral valve surgery.
-
Protocol number: MK-2140-011-00-NCT06890884
A Randomized, Open-label, Multicenter, Phase 2 Study Evaluating the Efficacy and Safety of Zilovertamab Vedotin (MK-2140) Plus R-CHP Versus Polatuzumab Vedotin Plus R-CHP in Treatment-naïve Participants With GCB Subtype of Diffuse Large B-cell Lymphoma (DLBCL)
-
Protocol number: MK-2400-01A-00
MK-2400-01A Substudy: A Phase 1/2, Open-label Umbrella Substudy of MK 2400-U01 Master Protocol to Evaluate the Safety and Efficacy of Ifinatamab Deruxtecan-based Treatment Combinations or Ifinatamab Deruxtecan Alone in Participants with Metastatic Castration -resistant Prostate Cancer (mCRPC) (IDeate-Prostate02)
-
Protocol number: MK-2870-005-GOG-3095
A Phase 3, Randomized, Active-controlled, Open-label, Multicenter Study to Compare the Efficacy and Safety of MK-2870 Monotherapy Versus Treatment of Physician s Choice in Participants With Endometrial Cancer Who Have Received Prior Platinum-based Chemotherapy and Immunotherapy (MK-2870-005/ENGOT-en23/GOG 3095)
-
Protocol number: MK-2870-011
A Phase 3, Randomized, Open-label Study Comparing Efficacy and Safety of Sacituzumab Tirumotecan (sac-TMT, MK-2870) as a Monotherapy and in Combination with Pembrolizumab (MK-3475) Versus Treatment of Physician’s Choice in Participants With Previously Untreated Locally Recurrent Unresectable or Metastatic Triple-Negative Breast Cancer Expressing PD-L1 at CPS Less than 10 (TroFuse-011)
-
Protocol number: MK-2870-033-5005-NCT06952504
A Phase 3 Randomized, Open-label, Multicenter Study to Compare the Efficacy and Safety of Sacituzumab Tirumotecan in Combination with Pembrolizumab Versus Pembrolizumab Alone as First-line Maintenance Treatment in Participants with Mismatch Repair Proficient Endometrial Cancer (TroFuse-033/GOG-3119/ENGOT-en29)
-
Protocol number: MK-3475-587
A Multicenter, Open label, Phase III Study to Evaluate the Long-term Safety and Efficacy in Participants who are Currently on Treatment or in Follow-up in Studies that Include Pembrolizumab
-
Protocol number: MK-5684-004
A Phase 3, Randomized, Open-label Study of MK-5684 Versus Alternative Abiraterone Acetate or Enzalutamide in Participants with Metastatic Castration-resistant Prostate Cancer (mCRPC) That Progressed On or After Prior Treatment with One Nextgeneration Hormonal Agent (NHA)
-
Protocol number: MK-7902-014-03
A Phase 3, Randomized Study to Evaluate the Efficacy and Safety of Pembrolizumab (MK-3475) + Lenvatinib (E7080/MK-7902) + Chemotherapy Compared with Standard of Care as First-line Intervention in Participants with Metastatic Esophageal Carcinoma
-
Protocol number: MK-8591B-060
A Phase 2b, Randomized, Active-Controlled, Open-Label Clinical Study to Evaluate a Switch to Islatravir (ISL) and Ulonivirine (ULO)
-
Protocol number: MK3475-587
A Multicenter, Open label, Phase III Extension Trial to Study the Long-term Safety and Efficacy in Participants with Advanced Tumors Who Are Currently on Treatment or in Follow-up in a Pembrolizumab Trial
-
Protocol number: MKC-CI-002
A Randomized, Double-Blind, Placebo-Controlled Phase 2/3 Study of the Efficacy and Safety of Treatment With MNKD-101 (Clofazimine Inhalation Suspension) in Combination with Guideline-Based Therapy in Patients with Pulmonary Nontuberculous Mycobacterial Infection (Part A) Followed by an Open-Label Extension (Part B)
-
Protocol number: MOG001
A randomized, double-blind, placebo controlled, multicenter, phase 3, pivotal study with an open-label extension period to evaluate the efficacy and safety of rozanolixizumab in adult participants with myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOG-AD)
-
Protocol number: MOVR-NCT99999999
Muscular Dystrophy Association Neuromuscular Observational Research (MOVR) Data Hub Protocol Protocol# MDACS1
-
Protocol number: MS-LINK-NEXTGEN
Next Generation Learning Health System for Multiple Sclerosis (Next-Gen MS): A prospective, cluster-randomized study evaluating the impact of feed-forward patient reported outcomes data to clinical teams managing adults living with MS in a learning health system for MS research.
-
Protocol number: MS-LINK-OUTCOMES
The MS Leadership and Innovation Network (MS-LINK ) Outcomes Study: A Comprehensive Prospective Longitudinal Assessment of Patient and Clinical Reported Outcomes in Multiple Sclerosis Patients across North America
-
Protocol number: MS202329-0010-NCT06710132
PROCEADE PanTumor: A Phase 1b/2, Multicenter, Open-Label Study of Anti-CEACAM5 Antibody-Drug Conjugate M9140 in Participants With Advanced Solid Tumors
-
Protocol number: MSK22-100-NCT05389293
An Open-Label, Multicenter, Single-Arm, Phase-2 Study of Single-Agent Mosunetuzumab (BTCT4465A, RO7030816) for the Treatment of Patients with Newly Diagnosed Follicular Lymphoma in Need of Systemic Therapy
-
Protocol number: MTX230-301-NCT06950385
A Phase 3, Multi-Site, Prospective, Randomized, Double-Blind, Placebo-Controlled Trial of eRapa to Improve Clinical Outcomes in Patients With Familial Adenomatous Polyposis
-
Protocol number: MYSPINE
Patient specific instrumentation guides versus standard freehand technique for posterior-instrumented spinal lumbar and sacral fusion: a retrospective comparative cohort study
-
Protocol number: NAILNASH
Post-transplant NASH protocol to assess the development of metabolic co-morbidities and disease recurrence in those transplanted for NASH cirrhosis
-
Protocol number: NATURALHISTORY-NCT00009243
Evaluation, Pathogenesis and Treatment of Patients with or at Risk for Cerebrovascular Disease (A Natural History/Disease Pathogenesis Protocol)
-
Protocol number: NBI-98854-HD3006
Open-Label Rollover Study for Continuing Valbenazine Administration for the Treatment of Chorea Associated with Huntington Disease
-
Protocol number: NCT01892345
A Randomized, Double-Blind, Placebo-Controlled, Multi-Center Trial To Evaluate The Safety And Efficacy Of Eculizumab In Patients With Relapsing Neuromyelitis Optica (NMO)
-
Protocol number: NCT02242487
A 12-Month, Dose-Level Blinded Study Investigating the Safety and Efficacy of CVT-301 (Levodopa Inhalation Powder) in Parkinsons Disease Patients With Motor Response Fluctuations (OFF Phenomena)
-
Protocol number: NEOD001-301-NCT04973137
A Phase 3, Randomized, Multicenter, Double-Blind, Placebo-Controlled, Efficacy and Safety Study of Birtamimab Plus Standard of Care vs. Placebo Plus Standard of Care in Mayo Stage IV Subjects with Light Chain (AL) Amyloidosis
-
Protocol number: NEOMATRIX
A Phase 2a, Open-Label Study of the Safety, Tolerability, and Pharmacokinetics of a Single Dose of cP12 in Otherwise Healthy Adults with Up To and Including 5% Total Body Surface Area Thermal Burns
-
Protocol number: NEOSURG-IIT-NCT06449313
A PHASE II SINGLE-ARM STUDY OF NEOADJUVANT CHEMO-IMMUNOTHERAPY AND SURGICAL RESECTION IN LOCALLY ADVANCED NON-SMALL CELL LUNG CANCER WITH CONTRALATERAL MEDIASTINAL LYMPH NODE INVOLVEMENT (NEO-SURG)
-
Protocol number: NEROFE
Phase I Study of NEROFE and Doxorubicin in KRAS-mutated ST2-positive Solid Tumors
-
Protocol number: NEXEONAVX
Safety, Efficacy, & Use of Decellularized Femoral Artery Allograft for Arteriovenous Access for Hemodialysis: A Multi-center Prospective Registry Study
-
Protocol number: NIC-NCT02720445
Long Term Nicotine Treatment of Mild Cognitive Impairment
-
Protocol number: NOFEAR-BE-NCT03554356
Safety and Efficacy of the CryoBalloon Ablation for Treatment of Patients with Resistant Barrett's Esophagus (BE) - The Resistant BE Trial (ReBET)
-
Protocol number: NOVARTISCLOU064C12302
A randomized, double-blind, double-dummy, parallel-group study, comparing the efficacy and safety of remibrutinib versus teriflunomide in participants with relapsing multiple sclerosis, followed by extended treatment with open-label remibrutinib
-
Protocol number: NOVOARTEMIS
ARTEMIS - Effects of ziltivekimab versus placebo on cardiovascular outcomes in patients with acute myocardial infarction
-
Protocol number: NRG-BR007-NCT04852887
A PHASE III CLINICAL TRIAL EVALUATING DE-ESCALATION OF BREAST RADIATION FOR CONSERVATIVE TREATMENT OF STAGE I, HORMONE SENSITIVE, HER2-NEGATIVE, ONCOTYPE RECURRENCE SCORE </= 18 BREAST CANCER
-
Protocol number: NRG-BR009-NCT05879926
A Phase III Adjuvant Trial Evaluating the Addition of Adjuvant Chemotherapy to Ovarian Function Suppression plus Endocrine Therapy in Premenopausal Patients with pN0-1, ER-Positive/HER2-Negative Breast Cancer and an Oncotype Recurrence Score ≤ 25 (OFSET)
-
Protocol number: NRG-CC005-FORTE-NCT05080673
Five or Ten Year Colonoscopy for 1-2 Non-Advanced Adenomatous Polyps (FORTE)
-
Protocol number: NRG-CC009
PHASE III TRIAL OF STEREOTACTIC RADIOSURGERY (SRS) VERSUS HIPPOCAMPAL-AVOIDANT WHOLE BRAIN RADIOTHERAPY (HA-WBRT) FOR 10 OR FEWER BRAIN METASTASES FROM SMALL CELL LUNG CANCER
-
Protocol number: NRG-CC015-NCT06748222
HARNESSING E-MINDFULNESS APPROACHES FOR LIVING-AFTER BREAST CANCER---HEAL-ABC
-
Protocol number: NRG-GI008-NCT05174169
Colon Adjuvant Chemotherapy Based on Evaluation of Residual Disease
-
Protocol number: NRG-GI011-NCT06958328
A PHASE III RANDOMIZED TRIAL OF DOSE ESCALATED RADIATION IN LOCALLY ADVANCED PANCREAS CANCER (LAPC) PATIENTS (LAP100)
-
Protocol number: NRG-GY-026
A Phase II/III Study of Paclitaxel/Carboplatin Alone or Combined With Either Trastuzumab and Hyaluronidase-oysk (HERCEPTIN HYLECTA) or Pertuzumab, Trastuzumab, and Hyaluronidase-zzxf (PHESGO) in HER2 Positive, Stage I-IV Endometrial Serous Carcinoma or Carcinosarcoma
-
Protocol number: NRG-HN009-NCT05050162
Comparing High-Dose Cisplatin Every Three Weeks to Low-Dose Cisplatin Weekly When Combined With Radiation for Patients With Advanced Head and Neck Cancer
-
Protocol number: NTX24301
A Phase 3, Multicenter, Randomized, Subject and Evaluator Blinded , Controlled Study Evaluating the Safety and Efficacy of NTX-001 Compared to Standard of Care (Neurorrhaphy) in the Treatment of Upper Extremity Transected Nerves Requiring Surgical Repair.
-
Protocol number: NUTRI-GUARD
A PHASE 3, PROSPECTIVE, MULTICENTER, DOUBLE-BLIND, RANDOMIZED, CONTROLLED, ADAPTIVE STUDY TO DEMONSTRATE THE SAFETY AND EFFICACY OF DEFENCATH® IN REDUCING CENTRAL LINE-ASSOCIATED BLOODSTREAM INFECTIONS (CLABSIS) IN ADULT PARTICIPANTS RECEIVING TOTAL PARENTERAL NUTRITION (TPN) VIA CENTRAL VENOUS CATHETER (CVC)
-
Protocol number: NUVA-PFC1020
A prospective, multicenter study evaluating the safety and performance of posterior fixation in trauma, reconstructive, and tumor surgery of the occipito-cervico-thoracic spine.
-
Protocol number: NUVA-PFC1020-WEINER
A prospective, multicenter study evaluating the safety and performance of posterior fixation in trauma, reconstructive, and tumor surgery of the occipito-cervico-thoracic spine.
-
Protocol number: NVG-2089-200
An Open-label, Intra-participant Dose Escalation Study to Evaluate the Safety, Tolerability, and Efficacy of Intravenous NVG-2089 in Participants with Immune Thrombocytopenia
-
Protocol number: NVL-330-01-NCT06521554
A Phase 1a/1b Study of the Selective Tyrosine Kinase Inhibitor NVL-330 in Patients with Advanced or Metastatic HER2-altered NSCLC (HEROEX) study
-
Protocol number: NVL-520-01
A Phase 1/2 Study of the Highly Selective ROS1 Inhibitor NVL-520 in Patients with Advanced NSCLC and Other Solid Tumors (ARROS-1)
-
Protocol number: NVL-655-01
A Phase 1/2 Study of the Selective Anaplastic Lymphoma Kinase (ALK) Inhibitor NVL-655 in Patients with Advanced NSCLC and Other Solid Tumors (ALKOVE-1)
-
Protocol number: OASIS-NCT06154005
OASIS: OsteoAdapt SP Feasibility Study A Prospective, Blinded, Controlled, Dose-Randomized Clinical Investigation of OsteoAdapt SP in Single-level Transforaminal Lumbar Interbody Fusion (TLIF) For The Treatment of Symptomatic Degenerative Disease of The Lumbosacral Spine
-
Protocol number: OCCLUFLEX-NCT05069558
Prospective randomized multi-center controlled clinical investigation comparing PFO outcomes of the Occlutech Flex II PFO Occluder to standard of care PFO occlusion
-
Protocol number: OG-6219-P001
A Phase 2a/b, Randomized, Double-blind, Placebo-controlled, Parallel Group, Multicenter, Clinical Study to Evaluate the Efficacy and Safety of OG-6219 in 3 Dose Levels, in Women 18 to 49 Years of Age with Moderate to Severe Endometriosis-related Pain
-
Protocol number: OHAND-NCT04035005
A PHASE IIIb MULTICENTER, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDY TO EVALUATE THE EFFICACY AND SAFETY OF OCRELIZUMAB IN ADULTS WITH PRIMARY PROGRESSIVE MULTIPLE SCLEROSIS
-
Protocol number: ONWARDS11-NN1436-8182
A 26-week Study Comparing the Efficacy and Safety of Once-weekly Insulin Icodec and Once-daily Insulin Glargine U100, Both in Combination with Insulin Aspart, in Adults with Type 1 Diabetes
-
Protocol number: OPT-RATE-AF
Optimal pacing Rate for cardiac resynchronization therapy after atrioventricular node ablation in persistent Atrial Fibrillation and heart failure
-
Protocol number: OPTIM-R
Optimizing Post-sTroke Intervention Methods for Recovery (OPTIM-R): Evaluating the impact of therapy content on motor recovery PRINCIPAL INVESTIGATOR:
-
Protocol number: ORIC-114-01-NCT05315700
An Open-Label, Phase 1/2 Study of ORIC-114 as a Single Agent or in Combination With Chemotherapy, in Patients With Advanced Solid Tumors Harboring an EGFR or HER2 Alteration
-
Protocol number: OSE2101C302-NCT06472245
A randomized, open-label, phase 3 trial comparing the efficacy and safety of OSE2101 versus docetaxel in HLA-A2 positive patients with metastatic Non- Small Cell Lung Cancer (NSCLC) and secondary resistance to Immune Checkpoint Inhibitor (ICI)- ARTEMIA study
-
Protocol number: OVARIAN-NCT05479045
A Combination Therapy Strategy to Prevent Anti-PD-1 Therapy Resistance in Metastatic Ovarian Cancer Patients
-
Protocol number: PACES
Anticoagulation for New-Onset Post-Operative Atrial Fibrillation after CABG (PACeS)
-
Protocol number: PARAFFIN
A pilot randomized controlled trial evaluating the effectiveness of paraffin wax on burn patient function and patient range of motion during rehabilitation regimens.
-
Protocol number: PCORINOSES
The NOSES study-NASAL STEROIDS, IRRIGATION, ORAL ANTIBIOTICS, AND SUBGROUP TARGETING FOR EFFECTIVE MANAGEMENT OF SINUSITIS
-
Protocol number: PE-TRACT
PULMONARY EMBOLISM; THROMBUS REMOVAL WITH CATHETER-DIRECTED THERAPY: THE PE-TRACT TRIAL
-
Protocol number: PESSARY
Application of Machine Learning Models for Predicting Pessary Fitting Success, Shape, and Size: An Observational Study
-
Protocol number: PHN-012-001-NCT07127874
First-in-Human, Phase 1 Study of PHN-012, an Antibody Drug Conjugate, in Patients With Advanced Solid Tumors
-
Protocol number: PIVATAL-NCT05034432
Prophylactic Intra-Operative Ventricular Arrhythmia Ablation in High-Risk LVAD Candidates (PIVATAL)
-
Protocol number: PIVOT-006
A Phase 3, Randomized Study of Adjuvant Cretostimogene Grenadenorepvec versus Observation for the Treatment of Intermediate Risk Non-Muscle Invasive Bladder Cancer (IR-NMIBC) Following Transurethral Resection of Bladder Tumor (TURBT)
-
Protocol number: PL101-HD301
A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Parallel Arm, Multicenter Study Evaluating the Efficacy and Safety of Pridopidine in Patients with Early Stage of Huntington Disease
-
Protocol number: PL101-HD501
Pridopidine Expanded Access Protocol in Patients with Huntington's Disease
-
Protocol number: POINTFORCE
POst-Market ClINical Follow-up STudy oF the SerranatOR PTA Serration Balloon CathEter
-
Protocol number: POLARIS
An Observational Study on the Role of Complement in Patients with Hematological Diseases
-
Protocol number: POMEGRANATE
The POMEGRANATE Trial A randomized controlled trial comparing pessary home management of Reia pessary versus standard of care pessary for treatment of pelvic organ prolapse
-
Protocol number: POSITIVELINKS
Pragmatic Efficacy Trial of mHealth to Improve HIV Outcomes in the DC Cohort
-
Protocol number: PPP001
A PHASE 3, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, MULTICENTER STUDY WITH OPEN-LABEL EXTENSION TO EVALUATE THE EFFICACY AND SAFETY OF BIMEKIZUMAB IN STUDY PARTICIPANTS WITH PALMOPLANTAR PUSTULOSIS
-
Protocol number: PRECIDENTD-GSM
PREvention of CardIovascular and DiabEtic kidney disease in Type 2 Diabetes.
-
Protocol number: PRECIDENTD-UMH
PREvention of CardIovascular and DiabEtic kidney disease in Type 2 Diabetes.
-
Protocol number: PRECISE
Perfusion imaging to identify posterior circulation candidates for thrombectomy
-
Protocol number: PRECISE-NCT05037968
A Prospective, Randomized, multi-center study to assess the performance of MagnEtOsTM Flex Matrix Compared to Cellular Allograft in patients undergoing up to four-level Instrumented poSterolatEral lumbar/thoraco-lumbar Fusion (PLF)
-
Protocol number: PRELUDE-IIT-NCT06798558
Phase II Study of Neoadjuvant Lu-177PSMA-617 in Patients with High Risk Localized Prostate Cancer Undergoing Radical Prostatectomy
-
Protocol number: PRELUDE-NCT02768402
Percutaneous Mitral Valve Replacement EvaLuation Utilizing IDE Early Feasibility Study (PRELUDE)
-
Protocol number: PRESERVE-HD
PReSeRVE-HD: PRospective, Multicenter, Observational Study of the Merit HeRO® Graft and Super HeRO® EValuated in End-Stage Renal Disease Patients on HemoDialysis
-
Protocol number: PREVAIL
Obicetrapib and Cardiovascular Outcomes: A Placebo-Controlled, Double-Blind, Randomized Phase 3 Study to Evaluate the Effect of 10 mg Obicetrapib in Participants With Atherosclerotic Cardiovascular Disease (ASCVD) Who are Not Adequately Controlled Despite Maximally Tolerated Lipid-Modifying Therapies
-
Protocol number: PREVAILGLOBAL
A randomized controlled study of the Prevail Drug-Coated Balloon in subjects with in-stent restenosis and a single arm prospectively enrolled study of the Prevail Drug-Coated Balloon for de novo lesions in small vessel disease
-
Protocol number: PRO00075142/STUDY00007172
REmote symptom COllection to improVE postopeRative care (RECOVER)
-
Protocol number: PRO2020-0369
A Phase 2B study of selinexor (KPT-330), in combination with carfilzomib, daratumumab or pomalidomide in patients with multiple myeloma relapsing on current therapy
-
Protocol number: PRO2022-0016-NCT05715229
A MULTI-CENTER PHASE II RANDOMIZED TRIAL OF IMMUNOTHERAPY VERSUS CHEMOIMMUNOTHERAPY GUIDED BY CIRCULATING TUMOR DNA BASED MOLECULAR RESPONSE ON PATIENTS WITH METASTATIC NSCLC
-
Protocol number: PROACTIVEHF-NCT04089059
A Prospective, Multi-Center, Open Label, Single Arm Clinical Trial Evaluating the Safety and Efficacy of the Cordella Pulmonary Artery Sensor System in New York Heart Association (NYHA) Class III Heart Failure Patients
-
Protocol number: PROACTIVEHF2-NCT05934487
A Prospective, Multi-Center, Open Label, Randomized Control Clinical Trial Evaluating the Safety and Efficacy of the Cordella™ Pulmonary Artery Sensor System in New York Heart Association (NYHA) Class II Heart Failure Patients
-
Protocol number: PROGEM-NCT05827055
Role of Cholecystokinin Receptor Blockade on the Tumor Microenvironment in Pancreatic Cancer
-
Protocol number: PROGRESS-NCT03777059
A PHASE 3, MULTICENTER, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, PARALLEL-GROUP STUDY TO EVALUATE THE EFFICACY, SAFETY, AND TOLERABILITY OF ATOGEPANT FOR THE PREVENTION OF CHRONIC MIGRAINE (PROGRESS)
-
Protocol number: PROSPECT-LUNG-NCT06632327
Perioperative Versus Adjuvant Systemic Therapy in Patients With Resectable Non-Small Cell Lung Cancer - PROSPECT LUNG
-
Protocol number: PROTECTH2H
Protect the Head to Head: A Safety and Efficacy Assessment of the Emboliner® Embolic Protection Device
-
Protocol number: PRP
Vaginal Injection of Platelet Rich Plasma for the Improvement of Sexual Function (V.I.P. Study)
-
Protocol number: PSR-APV
Aortic, Peripheral & Venous (APV) Product Surveillance Registry Platform Base
-
Protocol number: PTK0796-NTM-20203
A Phase 2, Double-Blind, Randomized, Parallel-Group, Placebo-Controlled, Multi-Center Study to Evaluate the Efficacy, Safety, and Tolerability of Oral Omadacycline in Adult Subjects with Nontuberculous Mycobacterial (NTM) Pulmonary Disease Caused by Mycobacterium abscessus Complex (MABc)
-
Protocol number: PUMA-ALI-4201-NCT06095505
A PHASE 2 STUDY OF ALISERTIB IN PATIENTS WITH EXTENSIVE STAGE SMALL CELL LUNG CANCER
-
Protocol number: R0000-HEMB-2187
A PROSPECTIVE STUDY TO EVALUATE DISEASE CHARACTERISTICS IN HEMOPHILIA B PARTICIPANTS RECEIVING PROPHYLAXIS WITH STANDARD OF CARE FIX REPLACEMENT THERAPY
-
Protocol number: RAGE-2021
RAGE inhibition to decrease cancer therapy related cardio toxicity in women with non-metastatic breast cancer
-
Protocol number: RC48G001-NCT04879329
A Phase 2 Multi-Cohort, Open-Label, Multi-Center Clinical Study Evaluating the Efficacy and Safety of Disitamab Vedotin (RC48-ADC) Alone or in Combination with Pembrolizumab in Subjects with Locally-Advanced Unresectable or Metastatic Urothelial Carcinoma That Expresses HER2
-
Protocol number: RECOVER-AUTONOMIC-NCT0630578
RECOVER-AUTONOMIC: A Platform Protocol for Evaluation of Interventions for Autonomic Dysfunction in Post-Acute Sequelae of SARS-CoV-2 Infection (PASC)
-
Protocol number: REGAIN
Regeneration of Acutely Injured Nerves with Temporary Electrical Stimulation (REGAIN)
-
Protocol number: REIMAGINE-WHC
A prospective, real-world, interventional study to evaluate the effect of mepolizumab on achieving clinical remission in participants with severe asthma.
-
Protocol number: REN007NCT06021457
A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Effect of RBT-1 on Reducing the Risk of Post-Operative Complications in Subjects Undergoing Cardiac Surgery
-
Protocol number: RESONANCE-NCT04687358
REgiStry Of the NAtural History of recurreNt periCarditis in pEdiatric and adult patients
-
Protocol number: RESTORE
RESTORE Study (REdo tranScatheter aortic valve replacement for Transcatheter aOrtic valve failuRE)
-
Protocol number: RESTORE-NCT02586623
RESTORE: A Clinical Study of Patients With Symptomatic Neurogenic Orthostatic Hypotension to Assess Sustained Effects of Droxidopa Therapy
-
Protocol number: RESTOREWHC
RESTORE Study (REdo tranScatheter aortic valve replacement for Transcatheter aOrtic valve failuRE)
-
Protocol number: RIMEGEPANT
Rimegepant as Preemptive Treatment for Predictable Trigger-Induced Migraine in Adult Patients in the US
-
Protocol number: RMC-LUNG-101-NCT06162221
A Platform Study of RAS(ON) Inhibitor Combinations in Patients With RAS-Mutated Non-Small Cell Lung Cancer (NSCLC)
-
Protocol number: ROADSTER3-NCT05365490
POST-APPROVAL STUDY of TRANSCAROTID ARTERY REVASCULARIZATION in STANDARD RISK PATIENTS with SIGNIFICANT CAROTID ARTERY DISEASE The ROADSTER 3 Study
-
Protocol number: ROCHE-WP44131
A PHASE I, MULTICENTER, OPEN-LABEL, UNCONTROLLED STUDY TO EVALUATE PHARMACOKINETICS, PHARMACODYNAMICS, SAFETY, AND TOLERABILITY OF SATRALIZUMAB IN ADULT PATIENTS WITH AQP4 ANTIBODY-POSITIVE NEUROMYELITIS OPTICA SPECTRUM DISORDER WITH BODYWEIGHT >100 KG
-
Protocol number: RP-2021-IST
A multicenter observational data registry for outcomes of inappropriate sinus tachycardia and postural orthostatic tachycardia syndrome treatment.
-
Protocol number: RP1-104-IGNYTE-3
A Randomized, Controlled, Multicenter, Phase 3 Clinical Study Comparing Vusolimogene Oderparepvec in Combination with Nivolumab Versus Treatment of Physician s Choice in Patients with Advanced Melanoma That Has Progressed on an Anti-PD-1 and an Anti-CTLA-4 Containing Treatment Regimen [IGNYTE-3]
-
Protocol number: RP2-202-NCT06581406
A Randomized, Phase 2/3, Open-Label Study to Investigate the Efficacy and Safety of RP2 in Combination with Nivolumab versus Ipilimumab in Combination with Nivolumab in Immune Checkpoint Inhibitor-Naïve Adult Patients with Metastatic Uveal Melanoma
-
Protocol number: RPL554-NCFB-220
A Phase II, Randomized, Double-Blind, Placebo-Controlled Study of Ensifentrine in Subjects with Non-Cystic Fibrosis Bronchiectasis
-
Protocol number: RPNI
Regenerative Peripheral Nerve Interfaces to Treat Painful Digit and Hand Neuromas After Amputation: A Randomized, Prospective Study
-
Protocol number: RRTCR1-LGGAIM1
Intravesical Lactobacillus for Urinary Symptoms Among People With NLUTD Who Use Indwelling Catheters AIM 1
-
Protocol number: RRTCR1-LGGAIM2
Intravesical Lactobacillus for Urinary Symptoms Among People With NLUTD Who Use Indwelling Catheters AIM 2
-
Protocol number: RT234-PAH-CL202
A Phase 2b, Open-label, Single Dose Study to Evaluated the Safety and Efficacy of RT234 on Exercise Parameters Assessed by Cardiopulmonary Exercise Testing (CPET) in Subjects With Pulmonary Arterial Hypertension (PAH)
-
Protocol number: RYZ101-301
Phase 1b/3 global, randomized, controlled, open-label trial comparing treatment with RYZ101 to standard of care (SoC) therapy in subjects with inoperable, advanced, somatostatin receptor expressing (SSTR+), well-differentiated gastro-enteropancreatic neuroendocrine tumors (GEP-NETs) that have progressed following prior 177Lu labelled somatostatin analogue (177Lu-SSA) therapy (ACTION-1)
-
Protocol number: S2209-NCT05561387
S2209: A PHASE III RANDOMIZED TRIAL FOR NEWLY DIAGNOSED MULTIPLE MYELOMA (NDMM) PATIENTS CONSIDERED FRAIL OR IN A SUBSET OF INTERMEDIATE FIT COMPARING UPFRONT THREE-DRUG INDUCTION REGIMENS FOLLOWED BY DOUBLE- OR SINGLE-AGENT MAINTENANCE.
-
Protocol number: S2213-NCT06022939
A Phase III, Randomized Study of Daratumumab, Cyclophosphamide, Bortezomib and Dexamethasone (Dara-VCD) Induction Followed by Autologous Stem Cell Transplant or Dara-VCD Consolidation and Daratumumab Maintenance in Patients With Newly Diagnosed AL Amyloidosis
-
Protocol number: S2303-NCT06203600
Randomized Phase II/III Trial of 2nd Line Nivolumab + Paclitaxel + Ramucirumab Versus Paclitaxel + Ramucirumab in Patients With PD-L1 CPS >= 1 Advanced Gastric and Esophageal Adenocarcinoma (PARAMMUNE)
-
Protocol number: S2308-NCT06337318
Randomized Phase III Study of Mosunetuzumab vs. Rituximab for Low Tumor Burden Follicular Lymphoma
-
Protocol number: SAFEKIDNEY
Safety, tolerability and efficacy of AntiBKV as treatment of BKV infection in kidney transplant recipients, a randomized phase II/III study, double-blind and placebo-controlled
-
Protocol number: SAGE324-ETD-202
A Phase 2, Double-Blind, Randomized, Placebo-Controlled, Dose-Response Study of SAGE-324 for the Treatment of Essential Tremor
-
Protocol number: SANOFI-EFC17504
A randomized, double-blind, Phase 3 study comparing efficacy and safety of frexalimab (SAR441344) to placebo in adult participants with nonrelapsing secondary progressive multiple sclerosis
-
Protocol number: SANOFI-EFC17919
Master protocol of two independent, randomized, double-blind, Phase 3 studies comparing efficacy and safety of frexalimab (SAR441344) to teriflunomide in adult participants with relapsing forms of multiple sclerosis
-
Protocol number: SANOFI-LTS17043
An interventional, Phase 3 extension study to investigate long-term safety and tolerability of tolebrutinib in participants with relapsing multiple sclerosis, primary progressive multiple sclerosis, or nonrelapsing secondary progressive multiple sclerosis
-
Protocol number: SANOFIACT16970
A Phase 2, multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of SAR443820 in adult participants with amyotrophic lateral sclerosis, followed by an open-label extension
-
Protocol number: SAVISCOUT
SAVI SCOUT® System for Excision of nonpalpable breast lesions: Single institutional study regarding experiences, lessons learned & comparisons to needle localization
-
Protocol number: SELUTION-DENOVO
A Prospective Randomized Single-Blind Multicenter Study to Assess the Safety and Effectiveness of the SELUTION SLR™ 014 PTCA Drug Eluting Balloon in the Treatment of Subjects with De Novo Coronary Lesions in Small Vessels
-
Protocol number: SELUTION-SLR-NCT04280029
SELUTION SLR™ 014 ISR: A Prospective Randomized Single Blind Multicenter Study to Assess the Safety and Effectiveness of the SELUTION SLR™ 014 Drug Eluting Balloon in the Treatment of Subjects with In-stent Restenosis
-
Protocol number: SELUTION4BTK
A Prospective Randomized Multicenter Single Blinded Study to Assess the Safety and Effectiveness of the SELUTION SLR™ 014 Drug Eluting Balloon in the Treatment of CLTI Patients with BTK Artery Lesions
-
Protocol number: SELUTION4SFA
A Prospective Randomized Multicenter Single Blinded Study to Assess the Safety and Efficacy of the SELUTION SLR™ 018 Drug Eluting Balloon in the Treatment of Subjects with Femoropopliteal Artery Lesions
-
Protocol number: SEPSIS-BURN
Early Diagnosis of Burn Sepsis Using Transcriptomic Signatures
-
Protocol number: SIMPLAAFY
WATCHMAN FLX™ Pro Left Atrial Appendage Closure Device with Alternative Post-Implant Monotherapy
-
Protocol number: SISTER
Social Interventions for Support during Treatment for Endometrial Cancer and Recurrence (SISTER): a multi-site randomized controlled trial
-
Protocol number: SJS-TENS
Comprehensive Genomic Analysis of Adverse Vaccine and Cutaneous Adverse Drug Reactions
-
Protocol number: SMA-CHART-REVIEW-NCT99999999
Emerging Real-World Use of Nusinersen in Adult Patients with Spinal Muscular Atrophy (SMA) in the US: A Multi-Site Chart Review Study
-
Protocol number: SMILE-AF
Posterior Wall Substrate Modification Using Irreversible Electroporation for the Treatment of Paroxysmal Atrial Fibrillation
-
Protocol number: SOLAR
Transvaginal Photobiomodulation for the Treatment of Dyspareunia in Endometriosis Patients: A Randomized Controlled Trial
-
Protocol number: SONOMYOGRAPHIC-ULP
Sonomyographic Upper Limb Prosthetics: A New Paradigm
-
Protocol number: SOS-AMI
Multi-center, double-blind, randomized, placebo-controlled, parallel-group study to evaluate the efficacy and safety of self-administered subcutaneous selatogrel for prevention of all-cause death and treatment of acute myocardial infarction in subjects with a recent history of acute myocardial infarction
-
Protocol number: SPI-62-CL-2006
Clofutriben And Placebo Phase 2 Trial Against INtractable Type 2 Diabetes (CAPTAIN-T2D)
-
Protocol number: SPLIT
Prospective Observational Study to Assess Outcomes in Pediatric Liver Transplant Recipients
-
Protocol number: SPYRALAFFIRM
The SPYRAL AFFIRM Global Clinical Study of Renal Denervation with the Symplicity Spyral Renal Denervation System in Subjects with Uncontrolled Hypertension (SPYRAL AFFIRM)
-
Protocol number: SRF201
Platform Clinical Study for Conquering Scleroderma: A Multicenter, Double-Blind, Randomized, Placebo-Controlled, Phase 2b Platform Clinical Study to Evaluate the Safety and Efficacy of Investigational Products in Participants with Interstitial Lung Disease Secondary to Systemic Sclerosis (CONQUEST)
-
Protocol number: STAGO-PV-ET
Biomarkers of Global Hemostasis in Polycythemia Rubra Vera and Essential Thrombocythemia
-
Protocol number: STAR-221
A Randomized, Open-Label, Multicenter Phase 3 Trial of Domvanalimab, Zimberelimab,and Chemotherapy Versus Nivolumab and Chemotherapy in Participants with Previously-Untreated Locally Advanced Unresectable or Metastatic Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma
-
Protocol number: STCM02
A Pivotal Study Evaluating the Safety and Efficacy of the ShiraTronics Migraine Therapy System in RELIEVing, Interrupting, and Preventing Chronic Migraine (RELIEV-CM2)
-
Protocol number: STEP-1
A Randomized, Multi-Center, Pivotal Efficacy and Safety Study Evaluating the EndoBarrier® System for Glycemic Improvement in Patients with Inadequately Controlled Type 2 Diabetes and Obesity, the STEP-1 Study
-
Protocol number: STK-012-101-NCT05098132
A Phase 1a/1b Study to Evaluate the Safety and Tolerability of STK-012 Monotherapy and in Combination With Pembrolizumab in Patients With Selected Advanced Solid Tumors
-
Protocol number: STML-ELA-0422-MWHC-NCT06492616
Elacestrant versus Standard Endocrine Therapy in Women and Men with Node-positive, Estrogen Receptor-positive, HER2-negative, Early Breast Cancer with High Risk of Recurrence A Global, Multicenter, Randomized, Open-label Phase 3 Study (ELEGANT)
-
Protocol number: STRO-002-GM3
A Phase 2 Open-label Study Evaluating the Efficacy and Safety of Luveltamab Tazevibulin (STRO-002) in Women with Relapsed Platinum-resistant Epithelial Ovarian Cancer (including Fallopian Tube or Primary Peritoneal Cancers) expressing Folate Receptor alpha (FOLR1)
-
Protocol number: STUDY00000017
A randomized, double blind, placebo-controlled study to evaluate the impact of Bosutinib on safety, tolerability, biomarkers and clinical outcomes in Dementia with Lewy Bodies (DLB)
-
Protocol number: STUDY00000122
A randomized, double blind, placebo-controlled study to evaluate the impact of Nilotinib treatment on safety, tolerability, pharmacokinetics and biomarkers in Dementia with Lewy Bodies (DLB)
-
Protocol number: STUDY00000196-NCT06554158
A Phase II Study of Post-Resection Radiation Dose and Treatment Site De-Escalation for Favorable Prognosis Human Papilloma Virus (HPV) or p16-Positive Oropharyngeal Cancer
-
Protocol number: STUDY00000197-NCT06902623
A Phase II, Single-Institution Study of Treatment De-Escalation for Favorable Prognosis, Stage I-II Human Papilloma Virus (HPV) or p16-Positive Oropharyngeal Cancer Receiving Definitive Radiotherapy
-
Protocol number: STUDY00000266
A randomized, double blind, placebo-controlled study to evaluate the impact of K0706 on safety, tolerability, pharmacokinetics and pharmacodynamics and clinical outcomes in Dementia with Lewy Bodies (DLB).
-
Protocol number: STUDY00000307
Identifying early neuroimaging biomarkers using diffusivity measures and functional connectivity to predict neurodevelopmental outcomes in neonates with hypoxic ischemic injury
-
Protocol number: STUDY00001137
Parent Communication Study IV: Improving Genetic Counseling for BRCA+ Mothers
-
Protocol number: STUDY00001487-TBCRC047
Innovative Combination Immunotherapy for Metastatic Triple Negative Breast Cancer (TNBC): A Multicenter, Multi-Arm Translational Breast Cancer Research Consortium Study
-
Protocol number: STUDY00002424
Investigating Exposure to Endocrine Disrupting Chemicals and Poor Sleep in Breast Cancer Survivors
-
Protocol number: STUDY00002789
CONTIGO - Informing Latinas About HBOC Risk: a Randomized Controlled Trial
-
Protocol number: STUDY00003077-NCT04520776
A multicenter, prospective, randomized, clinical trial comparing the safety and effectiveness of the BAGUERA® C Cervical Disc Prosthesis to the Mobi-C® Cervical Disc for the treatment of patients with symptomatic cervical disc disease at a single level.
-
Protocol number: STUDY00003267
Families SHARE: Development and evaluation of a family health history education toolkit
-
Protocol number: STUDY00003277
A mutlilevel intervention to address disparities in lung cancer screening
-
Protocol number: STUDY00003285
Use of MR Spectroscopy (MRS) to Differentiate Vertebral Metastases from Atypical Vertebral Hemangiomas
-
Protocol number: STUDY00003342
Longitudinal investigation of sociocultural and behavioral influences on symptom management, biological response, and functioning between Chinese and White breast cancer survivors
-
Protocol number: STUDY00003539
Improving Communication and Adherence in Black Breast Cancer Survivors
-
Protocol number: STUDY00003673
Developing a Multilevel Intervention for Screening Breast MRI
-
Protocol number: STUDY00003788
Identifying early neuroimaging biomarkers using diffusivity measures and functional connectivity to predict neurodevelopmental outcomes in neonates with prenatal opioid exposure
-
Protocol number: STUDY00004071
An advanced functional MRI study of frontostriatal injury in adults with HIV
-
Protocol number: STUDY00004397
Scaling Social Determinants of Health Screening, Social Support and Anti-Racism Training to Reduce Inequities in Minority Cancer Survivor Health and Wellbeing in Washington, DC
-
Protocol number: STUDY00004914
Providing Tobacco Treatment to Patients Undergoing Lung Cancer Screening at MedStar Health: Pilot Study
-
Protocol number: STUDY00005085
Examining the relationship between social and molecular risk factors for Black patients with endometrial cancer
-
Protocol number: STUDY00005472-ISTPROTOCOL
Istradefylline Effect on Parkinson’s Disease Tremor, Motor Symptoms and Non-motor Symptoms
-
Protocol number: STUDY00005505
A double blind, randomized, placebo-controlled trial evaluating the efficacy and safety of BI 1015550 over at least 52 weeks in patients with Idiopathic Pulmonary Fibrosis (IPF)
-
Protocol number: STUDY00005554
Peripheral and central contributions to auditory temporal processing deficits and speech understanding in older cochlear implantees
-
Protocol number: STUDY00006070
Providing Tobacco Treatment to Patients Undergoing Lung Cancer Screening at MedStar Health: A Randomized Trial
-
Protocol number: STUDY00006301
Feasibility and acceptability of a prototype device to improve insomnia in young adult cancer survivors
-
Protocol number: STUDY00006573
Glucose-Guided Eating (GGE) to reduce chronic disease risk: a pilot study
-
Protocol number: STUDY00006715-GENETICS
Understanding Communication Practices and Support Needs around Genetic Testing among Black Breast Cancer Patients Families
-
Protocol number: STUDY00006757
Personalized Oncology Promoting Equity for Black Lives (PROPEL)
-
Protocol number: STUDY00006772
Pridopidine Expanded Access Program (EAP) for Huntington Disease
-
Protocol number: STUDY00006794
Longitudinal assessment of benefits and harms of cannabis use among cancer patients during cancer treatment
-
Protocol number: STUDY00006942-NCT00000000
SGLT-2 Inhibitors for Thiazide-Induced Hyponatremia
-
Protocol number: STUDY00007140
Leveraging Telehealth to Improve Oral Health and Systemic Outcomes among Cancer Survivors
-
Protocol number: STUDY00007350
Assessing the feasibility of web-based insomnia treatment among prostate cancer survivors
-
Protocol number: STUDY00007454
Population-based assessment of patient-reported outcomes in adults living with metastatic colorectal cancer
-
Protocol number: STUDY00007558
Interventions for Managing Parenting and Cancer Team (IMPACT) 2.0
-
Protocol number: STUDY00007772
Decision-Making and Quality of Life Surrounding Hematologic Disease and Gene Therapy
-
Protocol number: STUDY00007859
Pilot Study of Use of Website about Radioactive Iodine Treatment in Patients with Intermediate-Risk Thyroid Cancer
-
Protocol number: STUDY00007935
Patients' Experiences with Cancer Pain: A Qualitative Exploration
-
Protocol number: STUDY00007953
Barriers to follow-up care among adolescent and young adult cancer survivors
-
Protocol number: STUDY00007990
Developing a Culturally Targeted Narrative Video about Skin Cancer Prevention for Hispanic Outdoor Workers
-
Protocol number: STUDY00008048
Retrospective Review of Palliative Consultation for Patients with Cancer
-
Protocol number: STUDY00008530
The FYI on MRI: A Multilevel Decision Support Intervention for Screening Breast MRI
-
Protocol number: STUDY00008549
The Role of Family Communication in Shared Behavioral and Metabolic Obesity-Related Cancer Risk Factors among Breast and Endometrial Cancer Survivors and At-Risk Chosen Partners
-
Protocol number: STUDY00009043
Addressing Clinical Trials Participation in an Oncology Program at Georgetown University
-
Protocol number: STUDY00009412
Effects of a Brief Educational Video on Breast MRI Knowledge
-
Protocol number: STUDY00009587
ICanPlay Pilot Study: Tailoring a Remote-Based Physical Activity Intervention for Children Ages 4-12 Years Undergoing Cancer Treatment
-
Protocol number: STUDY00009847-NCT05907564
Assessing the Safety and Efficacy of the treatment for Acute Pulmonary Embolism using the Inquis Medical Aventus Thrombectomy System (AVENTUS) - Sub-study
-
Protocol number: SUN-PRO00038942
Clinical Trial for Surgery of the Ulnar Nerve (SUN) at the Elbow
-
Protocol number: SUPERNUS-ONAPGO-830P401
Real-World Patient Experiences Using Continuous Subcutaneous Apomorphine Infusion (ONAPGOTM) in the United States: A Prospective, Phase 4, Multicenter, Observational Study in Parkinson's Disease
-
Protocol number: SURPLUS
Surgical Resection of Prosthetic Valve Leaflets Under Direct Vision (SURPLUS) Registry
-
Protocol number: SYN-Q
Quantification of phosphorylated alpha-synuclein in patients with Parkinsons disease and REM sleep behavior disorder (Syn-Q)
-
Protocol number: SYNEX
Efficacy of SynEx wound rinse in civilian surrogates of combat injury wounds.
-
Protocol number: T-MMFLEX-001
PROSPECTIVE EVALUATION OF MICROMATRIX® FLEX IN WOUNDS COMPLICATED BY TUNNELING AND UNDERMINING.
-
Protocol number: TAK-079-3002
A Phase 3, Randomized, Double-blind, Placebo-controlled Study to Evaluate Efficacy and Safety of Mezagitamab Subcutaneous Injection in Participants with Chronic Primary Immune Thrombocytopenia
-
Protocol number: TAK-113-4002
A Single Arm Phase 4 Trial to Evaluate the Safety and Efficacy of Oral Fruquintinib in the Treatment of Refractory Metastatic Colorectal Cancer in Patients from Minority Populations Underrepresented in Prior Fruquintinib Studies
-
Protocol number: TAPESTRY-NCT06287853
Multi-Center, Prospective Post Market Clinical Follow-Up Evaluating Arthroscopic Rotator Cuff Repair Augmented with TAPESTRY® Biointegrative Implant
-
Protocol number: TARGET-ALS
Target ALS Biomarker Study: Longitudinal Biofluids, Clinical Measures, and At-Home Measures
-
Protocol number: TARGET-LD-GU
An Observational Study of Patients with Chronic Liver Disease
-
Protocol number: TARGET-LD-WHC
An Observational Study of Patients with Chronic Liver Disease
-
Protocol number: TBCRC046-NCT04841148
A Phase II Trial of Avelumab or Hydroxychloroquine with or without Palbociclib to Eliminate Dormant Breast Cancer ( PALAVY )
-
Protocol number: TBCRC059-NCT05501704
ETHAN: A Phase II Study Comparing Different Endocrine THerapies for mAle Breast caNcer
-
Protocol number: TBCRC064-NCT06533826
A Phase II Non-comparative Trial of Datopotamab Deruxtecan (Dato-DXd) or Trastuzumab Deruxtecan (T-DXd) in Patients With Metastatic HER2-low Breast Cancer After Progression on Prior Antibody Drug Conjugate Therapy
-
Protocol number: TBCRC066-NCT06551116
Quantitative Immunofluorescence and/or RT-qPCR for Measuring HER2 in HER2-low Metastatic Breast Cancer
-
Protocol number: TELLTALE
NHLBI Transmural Electrosurgery LeafLet Traversal And Laceration Evaluation (TELLTALE) BASILICA-TAVR Trial
-
Protocol number: TENAXLEVELNCT05983250
A Phase 3, Double-Blind, Randomized, Placebo-Controlled Study of Levosimendan in Pulmonary Hypertension Patients With Heart Failure With Preserved Left Ventricular Ejection Fraction (PH-HFpEF); LEVEL: LEVosimendan to Improve Exercise Limitation in Patients With PH-HFpEF.
-
Protocol number: TERP
Toxic Products of Combustion in Burn and Smoke Inhalation Injuries (TERP)
-
Protocol number: TEVA-TV56286-NDG-20039
Multi-centered, Double-blind, Randomized, Placebo-controlled, Parallel Group Phase 2 Study of TEV-56286 for the Treatment of Patients with Multiple System Atrophy
-
Protocol number: TG1101-RMS401
Evaluating efficacy when transitioning from a current Disease Modifying Therapy (DMT) to ublituximab (ENHANCE)
-
Protocol number: TG1101-RMS406
REal world experieNce with BRIUMVI® (ublituximAB-xiiy) treated patients: a Longitudinal rEgistry study (ENABLE)
-
Protocol number: TH-IBA-CTR-1003
Theratechnologies Inc. - A Prospective and Retrospective Observational Study of Multidrug-Resistant Patient Outcomes with and without Ibalizumab in a Real-World Setting: United States (PROMISE-US)
-
Protocol number: THUMB-CMC
Prospective Longitudinal Study of Non-Operative and Operative Thumb CMC Arthritis Interventions
-
Protocol number: THUMB-CMC-MEANS
Evaluating a Hand Therapy Digital Web-based Application for Thumb Carpometacarpal Arthritis
-
Protocol number: TIGER-001
A Global Post Market Evaluation of Terumo Aortic Endovascular Grafts
-
Protocol number: TIPI
A Proof-of Concept, Randomized, Controlled Study of Tuberculosis Immunization with BCG to Prevent Infection in Healthy Adults (TIPI trial)
-
Protocol number: TP-172-NCT07046832
Clinical Evaluation of an Intra-procedural 3D Needle Guidance Platform for Performing Percutaneous Liver, Kidney, and Soft Tissue Organ Tumor Biopsy as an Adjunct to Standard Image Guidance
-
Protocol number: TRAC-AF-GEN2
Tracking Results of Ablation and LAA Management to Combat AF Registry (TRAC-AF Registry)
-
Protocol number: TREATMS-NCT03500328
TRADITIONAL VERSUS EARLY AGGRESSIVE THERAPY FOR MULTIPLE SCLEROSIS (TREAT-MS) TRIAL
-
Protocol number: TRICAV-PIVOTAL
A prospective, multicenter, randomized, controlled clinical trial, to evaluate the safety and effectiveness of the TricValve® Transcatheter Bicaval Valve System® compared with standard medical therapy in subjects with symptomatic severe tricuspid regurgitation who are high-risk for cardiac surgery.
-
Protocol number: TRIOMPHE-NCT04471909
A Multi-Arm, Multi-Center, Non-Randomized, Prospective, Clinical Study to Evaluate the Safety and Effectiveness of the NEXUS Aortic Arch Stent Graft System in Treating Thoracic Aortic Lesions Involving the Aortic Arch
-
Protocol number: TTNS-NCT04350359
Transcutaneous Tibial Nerve Stimulation for Spinal Cord Injury Neurogenic Bladder
-
Protocol number: TVD-101-001H-NCT05440708
A Phase 1b/2 Multicenter, Open-label Study to Evaluate the Safety and Efficacy of TTI-101 as Monotherapy and in Combination in Participants with Locally Advanced or Metastatic, and Unresectable Hepatocellular Carcinoma
-
Protocol number: U-C-TPD-2022-01-FS
Randomized, Controlled, Open-Label, Parallel Group, Multi-Center, Prospective Phase 4 Study Comparing the Efficacy of Transforming Powder Dressing to NPIAP Recommended Standard of Care Therapies in Stage 2, 3 and 4 Pressure Injuries
-
Protocol number: U-C-TPD-2022-01-GSH
Randomized, Controlled, Open-Label, Parallel Group, Multi-Center, Prospective Phase 4 Study Comparing the Efficacy of Transforming Powder Dressing to NPIAP Recommended Standard of Care Therapies in Stage 2, 3 and 4 Pressure Injuries
-
Protocol number: U-C-TPD-2022-01-NRH
Randomized, Controlled, Open-Label, Parallel Group, Multi-Center, Prospective Phase 4 Study Comparing the Efficacy of Transforming Powder Dressing to NPIAP Recommended Standard of Care Therapies in Stage 2, 3 and 4 Pressure Injuries
-
Protocol number: U-C-TPD-2022-01-WHC
Randomized, Controlled, Open-Label, Parallel Group, Multi-Center, Prospective Phase 4 Study Comparing the Efficacy of Transforming Powder Dressing to NPIAP Recommended Standard of Care Therapies in Stage 2, 3 and 4 Pressure Injuries
-
Protocol number: UCL-CHDI-1
HDClarity: a multi-site cerebrospinal fluid collection initiative to facilitate therapeutic development for Huntington s disease
-
Protocol number: ULURU-BURN
Prospective Randomized Open Label Multicenter Phase IV Clinical Trial to Compare Transforming Powder Dressing (TPD) to Current Standard of Care (SOC) Dressing Therapies in Acute Partial Thickness Burn Wounds
-
Protocol number: URCC-19075-CIPN-NCT04888988
Testing the Effects of Exercise on Chemotherapy-Induced Peripheral Neuropathy
-
Protocol number: URCC-21038-DIRECT-NCT05364086
An Observational Research Study for Cancer Patients on Immune Checkpoint Inhibitors, DiRECT Study (DiRECT)
-
Protocol number: URCC-22063-LOTUS-NCT06073431
An Observational Research Study to Uncover Subtypes of Cancer Cachexia, LOTUS-CC Study (LOTUS-CC)
-
Protocol number: URCC18110CD-ENABLE-NCT04062552
Learning Collaborative Vs Technical Assistance in Delivering a Palliative Care Program to Patients With Advanced Cancer and Their Caregivers
-
Protocol number: VASOMUNE
A Randomized, Double-blind, Placebo-controlled, Phase 2a Multiple Ascending Dose Study to Examine the Safety, Tolerability and Efficacy of AV-001 in Patients Hospitalized with Confirmed Severe COVID-19 Disease
-
Protocol number: VENUS-HF
Superior VENa Caval OcclUSion in Subjects with Acute Decompensated Heart Failure (VENUS-HF): An Early Feasibility Study
-
Protocol number: VERIFY-NCT05338697
Validation of Early Prognostic Data for Recovery Outcome After Stroke for Future, Higher Yield Trials (VERIFY)
-
Protocol number: VERISMO
AN OBSERVATIONAL STUDY OF OCRELIZUMABTREATED PATIENTS WITH MULTIPLE SCLEROSIS TO DETERMINE THE INCIDENCE AND MORTALITY RATES OF BREAST CANCER AND ALL MALIGNANCIES (VERISMO STUDY)
-
Protocol number: VERTEX-VX24-548-108
A Phase 3b, Open-label, Single-arm Study Evaluating the Effectiveness and Safety of Suzetrigine As Part of Multimodal Therapy for Acute Pain After Laparoscopic Procedures of the Intraperitoneal or Retroperitoneal Cavities or Arthroscopic Orthopedic Procedures
-
Protocol number: VIB0551-P3-S1
A RANDOMIZED, DOUBLE-BLIND, MULTICENTER, PLACEBO-CONTROLLED PHASE 3 STUDY WITH OPEN-LABEL PERIOD TO EVALUATE THE EFFICACY AND SAFETY OF INEBILIZUMAB IN ADULTS WITH MYASTHENIA GRAVIS
-
Protocol number: VIM0423-401
AN OBSERVATIONAL STUDY OF INDIVIDUALS WITH ISOLATED DYSTONIA
-
Protocol number: VIPER
Vaginal Injection of Platelet Rich Plasma (PRP) for the Genitourinary Syndrome of Menopause (GSM)
-
Protocol number: VLX-1005-003
A RANDOMIZED, DOUBLE-BLIND, PHASE 2 PILOT STUDY OF VLX-1005 VERSUS PLACEBO IN PARTICIPANTS WITH SUSPECTED HEPARIN INDUCED THROMBOCYTOPENIA TREATED WITH BACKGROUND STANDARD OF CARE
-
Protocol number: VLX-1005-003-MEDSTAR
A RANDOMIZED, DOUBLE-BLIND, PHASE 2 PILOT STUDY OF VLX-1005 VERSUS PLACEBO IN PARTICIPANTS WITH SUSPECTED HEPARIN INDUCED THROMBOCYTOPENIA TREATED WITH BACKGROUND STANDARD OF CARE
-
Protocol number: VLX-301
A Randomized Double-Blind Placebo-Controlled Study to Evaluate the Efficacy and Safety of Volixibat in the Treatment of Cholestatic Pruritus in Patients with Primary Sclerosing.Cholangitis (VISTAS.
-
Protocol number: VLX-601
A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Volixibat in the Treatment of Cholestatic Pruritus in Patients with Primary Biliary Cholangitis (VANTAGE)
-
Protocol number: VLX-601-GU
A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Volixibat in the Treatment of Cholestatic Pruritus in Patients with Primary Biliary Cholangitis (VANTAGE)
-
Protocol number: VNS-21-07-NCT05489588
Evaluation of the GORE® VIAFORT Vascular Stent for Treatment of Symptomatic Iliofemoral Venous Obstruction
-
Protocol number: VS01-IIA-01
A Phase 2a, open-label, randomized, controlled, multicenter, proof of concept study, to assess the efficacy, safety and tolerability of VS-01 on top of standard of care, compared to standard of care alone, in adult patients with acute-on-chronic liver failure (ACLF) grades 1 and 2 and ascites
-
Protocol number: VT-305
A PHASE 3, PROSPECTIVE, RANDOMIZED, MULTICENTER, SINGLE-BLIND, CONTROLLED STUDY EVALUATING ARTERIOVENOUS FISTULA OUTCOMES WITH AND WITHOUT A PERIVASCULAR SIROLIMUS-ELUTING COLLAGEN IMPLANT (THE ACCESS 2 TRIAL)
-
Protocol number: VULVIE
A randomized controlled trial of Vulvar fractionated CO2-Laser therapy with and without concomitant topical clobetasol propionate 0.05% ointment for treatment of Vulvar lichen sclerosus
-
Protocol number: VX15/2503-11
SEMA4D Blockade Safety and Brain Metabolic Activity in Alzheimer s Disease (AD): A Phase 1b/2a, Multi-center, Randomized, Double-Blind, Placebo-Controlled Safety and Biomarker Study of Pepinemab Anti-SEMA4D Antibody in early-AD
-
Protocol number: VYXEOS
Phase IB/II of CPX-351 as maintenance therapy in AML patients ineligible for bone marrow transplantation
-
Protocol number: WF-1805CD-HN-STAR-NCT04208490
Implementation and Effectiveness Trial of HN-STAR
-
Protocol number: WF-1806-NCT03998202
Myopenia and Mechanisms of Chemotherapy Toxicity in Older Adults With Colorectal Cancer (M&M) (NCORP)
-
Protocol number: WN43194
A PHASE III, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, MULTICENTER STUDY TO EVALUATE THE EFFICACY, SAFETY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF SATRALIZUMAB AS MONOTHERAPY OR IN ADDITION TO BASELINE THERAPY IN PATIENTS WITH MYELIN OLIGODENDROCYTE GLYCOPROTEIN ANTIBODY-ASSOCIATED DISEASE (MOGAD)
-
Protocol number: WO44263-INAVO122
A PHASE III, MULTICENTER, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDY EVALUATING THE EFFICACY AND SAFETY OF INAVOLISIB IN COMBINATION WITH PHESGO VERSUS PLACEBO IN COMBINATION WITH PHESGO AS MAINTENANCE THERAPY AFTER FIRST LINE INDUCTION THERAPY IN PARTICIPANTS WITH PIK3CA MUTATED HER2 POSITIVE LOCALLY ADVANCED OR METASTATIC BREAST CANCER
-
Protocol number: WP44714
A PHASE I/II STUDY TO EVALUATE THE SAFETY, TOLERABILITY, PHARMACOKINETICS, PHARMACODYNAMICS, AND EFFICACY OF NXT007 IN PERSONS WITH SEVERE OR MODERATE HEMOPHILIA A
-
Protocol number: XB010-101-NCT06545331
A Dose-Escalation and Expansion Study of XB010 as a Single Agent and Combination Therapy in Subjects With Locally Advanced or Metastatic Solid Tumors
-
Protocol number: XB371-101-NCT07123103
A DOSE ESCALATION AND EXPANSION STUDY OF XB371 ADMINISTERED IN PARTICIPANTS WITH LOCALLY ADVANCED OR METASTATIC SOLID TUMORS
-
Protocol number: XL092-311-NCT06943755
A Phase 2/3, Multicenter, Randomized Open-Label Study of Zanzalintinib vs Everolimus in Participants With Previously Treated, Unresectable, Locally Advanced or Metastatic Neuroendocrine Tumors
-
Protocol number: XPF-010-301-WANG
A Randomized, Double-blind, Placebo-Controlled, Multicenter Phase 3 Study to Evaluate the Safety, Tolerability, and Efficacy of XEN1101 as Adjunctive Therapy in Focal-Onset Seizures
-
Protocol number: XPF-010-303
A Randomized, Double-blind, Placebo-Controlled, Multicenter, Phase 3 Study to Evaluate the Safety, Tolerability, and Efficacy of XEN1101 as Adjunctive Therapy in Primary Generalized Tonic-Clonic Seizures
-
Protocol number: YL201-INT-101-01-NCT05434234
A Phase 1, Multicenter, Nonrandomized, Open-Label, First-in-Human Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of YL201 in Patients With Advanced Solid Tumors
-
Protocol number: ZUCARA-ZT01-CL-2001
A Phase 2a, Randomized, Double-blind, Placebo-controlled, Multiple-dose, Crossover Study of the Effect of ZT-01 on Frequency of Nocturnal Hypoglycemia in Type 1 Diabetes Mellitus
All clinical trials
Our trials are carefully planned research studies that have led to important discoveries such as new drugs, procedures, and diagnostic approaches that make our lives better.
Volunteering in our trial offers potential benefits, including:
-
The opportunity to become involved in the testing of a new drug that may have potential to improve your condition
-
Close contact with the study team for management of your disease
-
Contributing to medical science, which may help others now and in the future
Frequently asked questions
-
Q: Why volunteer?
A: Does volunteering for a clinical trial make sense for you? The following information will help you to decide.
-
Q: What are clinical trials and why are they important?
A: Clinical trials are carefully planned research studies that have led to important discoveries such as new drugs, devices, procedures, biologics, and diagnostic approaches. These new discoveries make our lives better such as new medicines to treat cancer, diabetes, and other diseases. Some other words for clinical trials are research, survey, or experiment.
Know what you are getting into and don’t be afraid to ask questions. A clinical trial may or may not help you personally. The results from the study could help others who have a health problem. Taking part in research is voluntary. It is your decision!
-
Q: Why do we need clinical trials?
A: A series of clinical trials for each possible treatment must be done before the Food and Drug Administration (FDA) will approve a drug, procedure, or device. Many of the trials done at MedStar Health Research Institute are trials testing possible drugs or devices. A drug must be shown to be safe and useful for public use before the FDA can approve it.
From the lab bench to the drug store, developing a new drug is a long and expensive process. It is estimated to take about 10 years and $800 million to bring one new drug to market (R & D Directions, January 2002).
-
Q: Are clinical trials safe?
A: Protections have been put in place to safeguard your rights, safety, and privacy in research. Many layers of oversight include an institutional review board (ethical review board), the Food and Drug Administration (FDA), and periodic monitoring of study data by independent experts, patient advocates, community advisory board members, and community partners.
-
Screening trials test the best way to detect certain diseases or health conditions
-
Diagnostic trials determine better tests or procedures for diagnosing a particular disease or condition
-
Treatment trials test new treatments, new combinations of drugs, or new approaches to surgery
-
Quality of life trials explore and measure ways to improve the comfort and quality of life of people with a chronic illness
-
-
Q: Who can participate in a clinical trial?
A: People with the condition being studied or healthy people can volunteer to take part in a clinical trial. Each study has specific entry requirements such as age or a medical condition necessary for participation.
The doctor in charge of the study must review the volunteers' medical history to determine if he/she is eligible to participate based on strict study conditions called entry criteria.
-
Q: Who is in charge of a clinical trial?
A: A medical doctor is usually responsible for carrying out the study according to the study protocol. Before a clinical trial can begin to enroll volunteers, an Institutional Review Board (IRB) must approve it. The IRB is a committee made up of healthcare experts that review each study to protect patient rights and ensure the safety of research volunteers. The IRB also ensures the research is scientifically sound and is involved in the review of safety issues throughout the study.
-
Q: What is informed consent?
A: One of the documents the IRB must review and approve is called an Informed Consent Form. This is provided to volunteers who are interested in participating in a clinical trial program. The informed consent includes everything a volunteer needs to know about the study so that he/she can make an informed decision as to whether or not to participate. The process allows volunteers to ask questions. Volunteers are encouraged to take their time in making a decision before signing the consent and joining the study. You and your family should feel completely comfortable with your decision to participate before you sign the consent.
-
Q: What are the benefits to being in a clinical trial?
A: Once enrolled in a clinical trial, many volunteers find there are a number of potential benefits to participation. These can include:
-
The opportunity to become involved in the testing of a new drug that may have potential to improve your condition.
-
Close contact with the study team for management of your disease.
-
Contributing to medical science, which may help others now and in the future.
However, volunteers understand that there may not be any benefit to participating in a study.
-
-
Q: Why do we need diversity in a research?
A: Race, ethnicity, age, and other factors can affect how people respond to medicine.
-
Q: Why do many still feel uneasy about being part of a clinical trial?
A: The Tuskegee Syphilis Study and the cancer cells taken from Henrietta Lacks without her knowledge are two reasons for the continued distrust with the African American community. Today, there is low participation in clinical trials from African Americans. We may not know if certain treatments will be effective in this population.
-
Q: Will my health insurance pay for a clinical trial?
A: Federal law requires insurance companies to cover routine costs, which are things you need regardless of a clinical trial. If the study sponsor requires extra testing, they will typically cover the cost.
-
Q: Can I drop out of a clinical trial and still receive quality care?
A: You are in control. If you decide you do not want to participate anymore, you can withdraw at any time. You will receive the same high-quality care at MedStar Health. Your clinical team may ask to continue monitoring you for a period of time to assess effects of the treatment you have already received.
-
Q: Are clinical trials considered a last-ditch effort for patients?

A: Not true at all! Clinical trials allow access to treatments that are up and coming but not yet available to the general population.
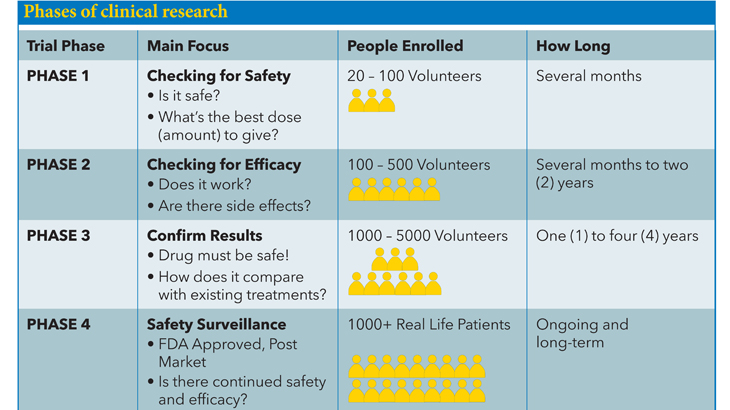
-
Q: Interested in participating in a clinical trial by MedStar Health?
A: Email JoinResearch@MedStar.net or call 833-998-0900 (toll-free) to learn more and ask about our open studies.










