Find a clinical trial
Please use one or more of the field below to begin your search.
All clinical trials
Our trials are carefully planned research studies that have led to important discoveries such as new drugs, procedures, and diagnostic approaches that make our lives better.
Volunteering in our trial offers potential benefits, including:
-
The opportunity to become involved in the testing of a new drug that may have potential to improve your condition
-
Close contact with the study team for management of your disease
-
Contributing to medical science, which may help others now and in the future
Frequently asked questions
-
Q: Why volunteer?
A: Does volunteering for a clinical trial make sense for you? The following information will help you to decide.
-
Q: What are clinical trials and why are they important?
A: Clinical trials are carefully planned research studies that have led to important discoveries such as new drugs, devices, procedures, biologics, and diagnostic approaches. These new discoveries make our lives better such as new medicines to treat cancer, diabetes, and other diseases. Some other words for clinical trials are research, survey, or experiment.
Know what you are getting into and don’t be afraid to ask questions. A clinical trial may or may not help you personally. The results from the study could help others who have a health problem. Taking part in research is voluntary. It is your decision!
-
Q: Why do we need clinical trials?
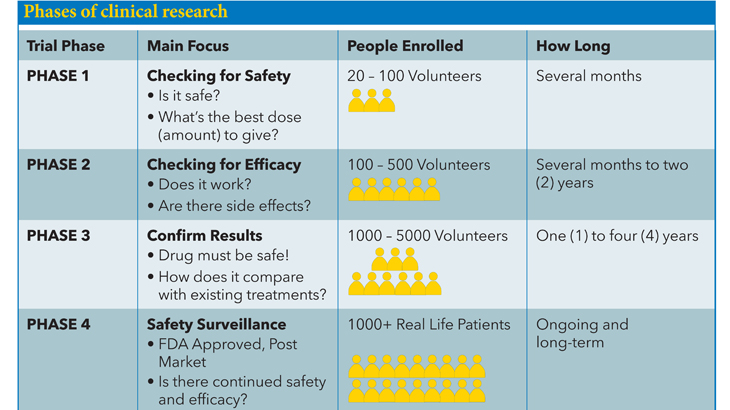
A: A series of clinical trials for each possible treatment must be done before the Food and Drug Administration (FDA) will approve a drug, procedure, or device. Many of the trials done at MedStar Health Research Institute are trials testing possible drugs or devices. A drug must be shown to be safe and useful for public use before the FDA can approve it.
From the lab bench to the drug store, developing a new drug is a long and expensive process. It is estimated to take about 10 years and $800 million to bring one new drug to market (R & D Directions, January 2002).
-
Q: Are clinical trials safe?
A: Protections have been put in place to safeguard your rights, safety, and privacy in research. Many layers of oversight include an institutional review board (ethical review board), the Food and Drug Administration (FDA), and periodic monitoring of study data by independent experts, patient advocates, community advisory board members, and community partners.
-
Screening trials test the best way to detect certain diseases or health conditions
-
Diagnostic trials determine better tests or procedures for diagnosing a particular disease or condition
-
Treatment trials test new treatments, new combinations of drugs, or new approaches to surgery
-
Quality of life trials explore and measure ways to improve the comfort and quality of life of people with a chronic illness
-
-
Q: Who can participate in a clinical trial?
A: People with the condition being studied or healthy people can volunteer to take part in a clinical trial. Each study has specific entry requirements such as age or a medical condition necessary for participation.
The doctor in charge of the study must review the volunteers' medical history to determine if he/she is eligible to participate based on strict study conditions called entry criteria.
-
Q: Who is in charge of a clinical trial?
A: A medical doctor is usually responsible for carrying out the study according to the study protocol. Before a clinical trial can begin to enroll volunteers, an Institutional Review Board (IRB) must approve it. The IRB is a committee made up of healthcare experts that review each study to protect patient rights and ensure the safety of research volunteers. The IRB also ensures the research is scientifically sound and is involved in the review of safety issues throughout the study.
-
Q: What is informed consent?
A: One of the documents the IRB must review and approve is called an Informed Consent Form. This is provided to volunteers who are interested in participating in a clinical trial program. The informed consent includes everything a volunteer needs to know about the study so that he/she can make an informed decision as to whether or not to participate. The process allows volunteers to ask questions. Volunteers are encouraged to take their time in making a decision before signing the consent and joining the study. You and your family should feel completely comfortable with your decision to participate before you sign the consent.
-
Q: What are the benefits to being in a clinical trial?
A: Once enrolled in a clinical trial, many volunteers find there are a number of potential benefits to participation. These can include:
-
The opportunity to become involved in the testing of a new drug that may have potential to improve your condition.
-
Close contact with the study team for management of your disease.
-
Contributing to medical science, which may help others now and in the future.
However, volunteers understand that there may not be any benefit to participating in a study.
-
-
Q: Why do we need diversity in a research?
A: Race, ethnicity, age, and other factors can affect how people respond to medicine.
-
Q: Why do many still feel uneasy about being part of a clinical trial?
A: The Tuskegee Syphilis Study and the cancer cells taken from Henrietta Lacks without her knowledge are two reasons for the continued distrust with the African American community. Today, there is low participation in clinical trials from African Americans. We may not know if certain treatments will be effective in this population.
-
Q: Will my health insurance pay for a clinical trial?
A: Federal law requires insurance companies to cover routine costs, which are things you need regardless of a clinical trial. If the study sponsor requires extra testing, they will typically cover the cost.
-
Q: Can I drop out of a clinical trial and still receive quality care?
A: You are in control. If you decide you do not want to participate anymore, you can withdraw at any time. You will receive the same high-quality care at MedStar Health. Your clinical team may ask to continue monitoring you for a period of time to assess effects of the treatment you have already received.
-
Q: Are clinical trials considered a last-ditch effort for patients?

A: Not true at all! Clinical trials allow access to treatments that are up and coming but not yet available to the general population.
-
Q: Interested in participating in a clinical trial by MedStar Health?
A: Email JoinResearch@MedStar.net or call 833-998-0900 (toll-free) to learn more and ask about our open studies.