MedStar Georgetown University Hospital becomes first hospital in DC metro area to use FDA-approved technology allowing for expanded access to organs that were previously deemed marginal
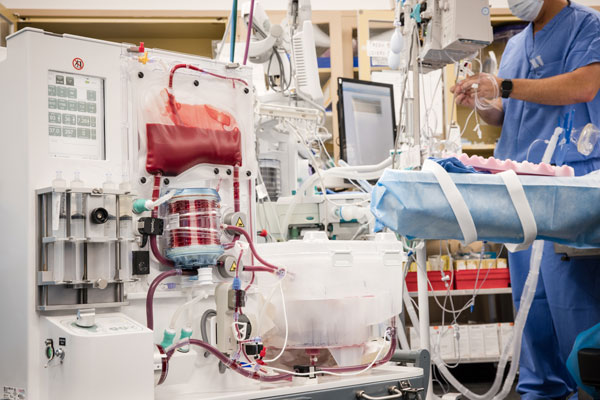
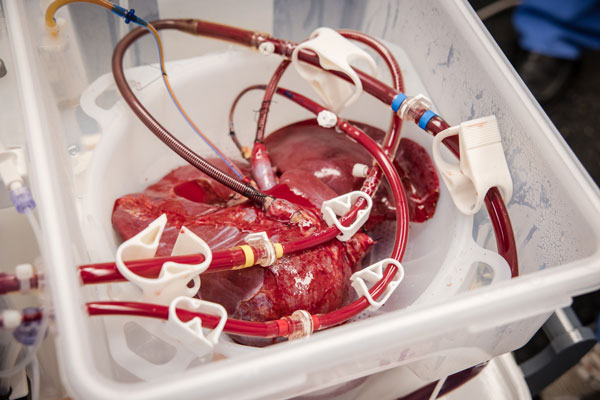
WASHINGTON – Transplant surgeons at MedStar Georgetown University Hospital are the first in the Washington Metropolitan area of the United States to successfully utilize a new FDA-approved technology, the OrganOx metra, which simulates connecting a liver to its recipient, allowing the donated liver to be preserved for up to 12 hours before being transplanted into the recipient. Our surgeons were also the first in the U.S. to enroll a patient in a new post-FDA approval study using the OrganOx metra. Transplant surgeons can now preserve and transplant organs that previously may have been deemed marginal or unusable, thus expanding the organ pool and extending access to thousands of people waiting for a liver transplant.
“This dynamic new technology allows us to provide patients in need of a liver transplant with a better-preserved organ and allows our transplant specialists to more thoroughly assess the quality and function of the donor organs while they are still attached to the device before transplant,” said Thomas M. Fishbein, MD, executive director, Medstar Georgetown Transplant Institute. “This technology allows us to increase the number of donor livers available for transplant, expanding access to life-saving transplants for the nearly 12,000 people in the US who are currently on the waiting list for a liver transplant.”
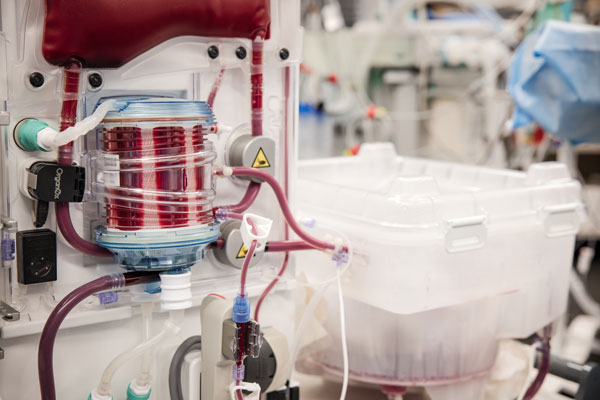
Rather than preserving the liver by simply cooling it to slow down metabolism, this new technology simulates connecting the organ to its recipient, providing oxygenated blood, medications, and nutrients at normal body temperature and comparable physiological pressures and flows. This allows the liver to function like it does while in the body, producing bile, metabolizing glucose, and maintaining pH levels. Having this new technology will allow transplant surgeons at MedStar Georgetown University Hospital to constantly assess the function of the organ prior to transplanting the liver into the recipient.
After being removed from the donor, the donated liver is connected to a centrifugal pump, which draws blood from the liver and varies in speed to mimic regular changes in blood pressure. That blood is then pumped into an oxygenator, where it is warmed and mixed with oxygen. The warmed, oxygenated blood is stored in a container and supplied to the liver in a flow similar to the way a person’s body sends blood to the liver. That blood is pumped back into the liver along with the necessary medicines and nutrients to keep the organ healthy.
Livers maintained on the OrganOx metra have been shown to suffer less injury than livers transported in cold storage in a randomized clinical trial. The study demonstrated an improvement shown by a reduction in the incidence of early allograft dysfunction, a measure of how much damage the organ sustains during the transplantation process, from 25% with cold storage to 19% with the OrganOx metra. Livers were also stored for 75% longer on the OrganOx metra compared to cold storage. The device demonstrated a comparable safety profile to cold storage. In another randomized European trial, donor liver preservation and assessment with the OrganOx metra resulted in over 50% fewer livers being discarded and 20% more successfully transplanted livers, compared with static cold storage1.
1 Nasralla D et al. Nature 2018; 557(7703):50-56
Categories
Media Contact
Matthew Holzapfel
571-302-6476 (mobile)
matthew.m.holzapfel@medstar.net