Find care now
If you are experiencing a medical emergency, please call 911 or seek care at an emergency room.

When conducting research, some populations of research participants require special considerations. The Code of Federal Regulations (CFR 45 part 46) Protection of Human Subjects includes Subparts B, C and D, that describe special protections and criteria for inclusion of pregnant women, prisoners and children. These protections are meant to safeguard of rights, welfare, and safety of these participants but does not mean they should automatically be excluded from research.
• Pregnant women, human fetuses, and neonates (Subparts B): Because research may pose additional and/or unknown risks to pregnant women, human fetuses and neonates, the regulations require additional safeguards in research. It is important to include pregnant women in research, as their exclusion from research creates a wider gap in understanding and knowledge.
• Prisoners (Subparts C): Because prisoners may not be free to make a truly voluntary and uncoerced decision regarding research participation, the regulations require additional safeguards for the protection of prisoners. For example: In order for an IRB to approve research involving prisoners the membership of the Board must include one or more prisoner representatives and that representative must be involved in the review of the research.
• Children (Subparts D): The CFR defines children as “persons who have not attained the legal age for consent to treatments or procedures involved in the research, under the applicable law of the jurisdiction in which the research will be conducted.” It is important to include, where appropriate, children as part of a research study. Children of all ages present different disease manifestations than adults, have different pharmacokinetics/ pharmacodynamics than adults and have a different psychology/psychiatry as part of their developing brain.
While the populations afforded special protections have traditionally been labeled “vulnerable” it should be noted that they are not specifically defined as such in Sub Parts B, C and D. The common rule does not define the term “vulnerable population”. The existence of additional protections should not specifically discourage inclusion of these populations in research. Rather, the protections are intended to guide the inclusion of these populations in such a way as to protect the rights and welfare of the individuals.
Although the regulation does not define the term vulnerable they do provide examples of research subjects “that are likely to be vulnerable to coercion or undue influence.” This is different from the special populations traditionally referred to as “vulnerable populations” description of sub parts B, C, and D. While children and prisoners are included in the current list of examples, pregnant women are no longer included as of the 2018 Revised Common Rule. The types of study populations that are likely to be vulnerable to coercion or undue influence may including but are not necessarily limited to:
• Children/minors
• Prisoners
• Employees
• Military persons and students/trainees in hierarchical organizations
• Terminally ill, comatose, physically and intellectually challenged individuals
• Institutionalized, elderly individuals
• Ethnic minorities
• Refugees
• Economically and educationally disadvantaged
When some or all participants are likely to be vulnerable to coercion or undue influence the regulations mandated that the IRB ensure “…additional safeguards have been included in the study to protect the rights and welfare of subjects.”
As previously noted, there has been an historical categorization of pregnant women and women of reproductive potential as a “vulnerable population.” While Sub Part B of the Common Rule describes special protections for pregnant women there is nothing about pregnancy, in and of itself, that renders a woman susceptible to coercion or undue influence. This categorization typically results in a tendency to exclude women, particularly pregnant women, from research. This exclusion may be intended to protect women from potential risk or may be done out of a misunderstanding of the special protection’s provision. Rather than serving to protect women from risk this broad exclusion of women from research has had a detrimental impact. Instead of shielding women and their fetuses from adverse effects, exclusion from clinical research in which they may be able to safely participate has served to limit understanding of pharmacokinetic and pharmacodynamic differences in women’s responses to treatment. Without scientific evidence this has drove the medical community to make potentially faulty assumptions about safety and efficacy of therapeutics when used with pregnant women.
While it is important to protect human subjects in research from coercion, undue influence and unjustified risks, it is equally important to ensure equitable selection of research subjects. This includes (but is not limited to) the inclusion of pregnant women and women of reproductive potential in research. Broad exclusion of any population, absent regulatory restriction or legitimate safety concerns, can serve to create a knowledge gap around the appropriate treatment modalities, appropriate dosing and the potential need to modify treatment modalities for some populations.
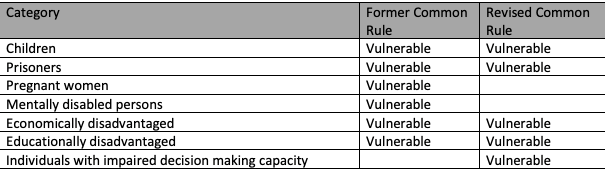
The table below includes the populations used as exemplars in the common rule for subjects that “…likely to be vulnerable to coercion or undue influence…” The table includes a notation for those included as examples in the pre-2018 Common Rule (Former Common Rule) and those now listed in the 2018 Revised Common Rule.
If you have any questions or concerns, please contact MHRI’s Office of Research Integrity Director, Jim Boscoe, at James.H.Boscoe@medstar.net.














