Featured News
-
February 03, 2026
Philanthropist Genevieve L. Murphy offers a new gift to the J.D. Murphy Jr. Cardio-Oncology fellowship, named for her late husband. COLUMBIA, Md —MedStar Health is pleased to announce the contin...
Browse news by hospital and team
For reporters
Share this
All News
-
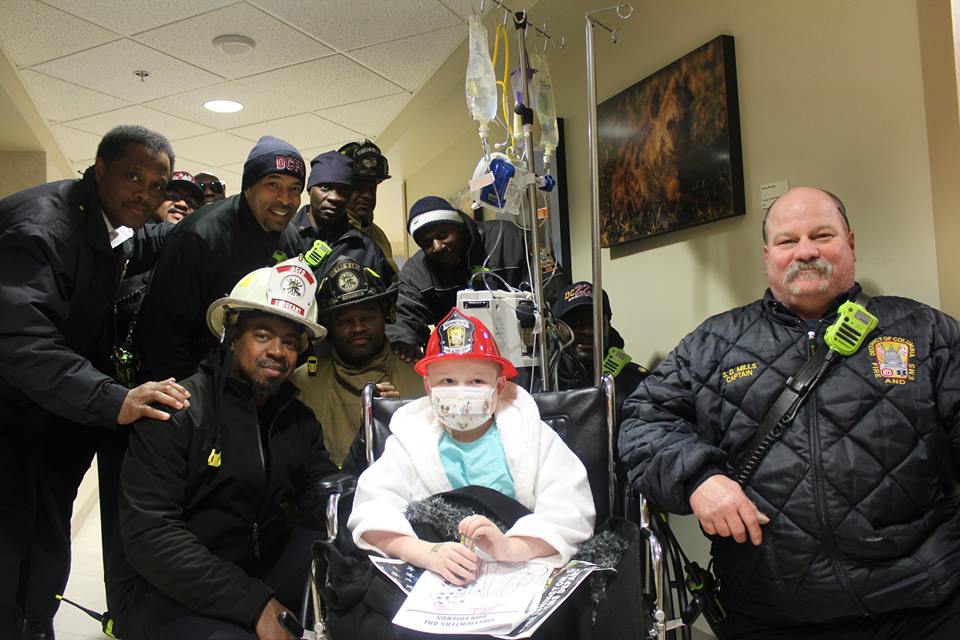 January 20, 2017
January 20, 2017Surprise Visit: D.C. Fire and EMS Encourage Pediatric Transplant Recipient D.C. Fire and Emergency Medical Services (D.C. Fire and EMS) surprised eight-year-old Hunter Scarborough, a transplant ...
-
January 18, 2017First Hospital in Maryland to Perform TAVR on Low Risk Patient
-
 January 17, 2017
January 17, 2017COLUMBIA, Md. (Nov. 17, 2017)— K. Eric De Jonge, MD, executive director of MedStar Health’s Medical House Call Program and director of Geriatrics at MedStar Washington Hospital Center, has been elect...
-
January 17, 2017Andrew Lincoln, ScD collaborated with researchers from US Lacrosse Sports Science to track the injuries in varsity men's lacrosse at the college level.
-
January 15, 2017
Led by Waddah B. Al-Refaie, MD, FACS, researchers from the MedStar Georgetown Surgical Outcomes Research Center and Georgetown University have found that the expansion of the New York State Medicaid i...
-
 January 15, 2017
January 15, 2017MedStar Heart & Vascular Institute at MedStar Union Memorial Hospital is one of only 80 cardiac centers in the world participating in a clinical research study of the transcatheter approach to r...
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
- 93
- 94
- 95
- 96
- 97
- 98
- 99
- 100
- 101
- 102
- 103
- 104
- 105
- 106
- 107
- 108
- 109
- 110
- 111
- 112
- 113
- 114
- 115
- 116
- 117
- 118
- 119
- 120
- 121
- 122
- 123
- 124
- 125
- 126
- 127
- 128
- 129
- 130
- 131
- 132
- 133
- 134
- 135
- 136
- 137
- 138
- 139
- 140
- 141
- 142
- 143
- 144
- 145
- 146
- 147
- 148
- 149
- 150
- 151
- 152
- 153
- 154
- 155
- 156
- 157
- 158
- 159
- 160
- 161
- 162
- 163
- 164
- 165
- 166
- 167
- 168
- 169
- 170
- 171
- 172














